推薦產品
形狀
powder
反應適用性
core: iridium
reagent type: catalyst
reaction type: Photocatalysis
活化光觸媒
465 nm
SMILES 字串
F[P-](F)(F)(F)(F)F.CC1=CC([Ir+]([N]2=C3C=C(C)C=C2)(C4=C5C=CC(C)=C4)([N]6=C7C=C(C(C)(C)C)C=C6)([N]8=C7C=C(C(C)(C)C)C=C8)[N]9=C5C=C(C)C=C9)=C3C=C1
應用
(Ir[Me(Me)ppy]2(dtbpy))PF6 or Ir(dmppy)2(dtbbpy)PF6 is an iridium photoredox catalyst that facilitates a variety of transformations using visible light, including the α- and β-alkylation of aldehydes.[1][2]
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
相關產品
產品號碼
描述
訂價
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
客戶也查看了
Zachary C Litman et al.
Nature, 560(7718), 355-359 (2018-08-17)
Living organisms rely on simultaneous reactions catalysed by mutually compatible and selective enzymes to synthesize complex natural products and other metabolites. To combine the advantages of these biological systems with the reactivity of artificial chemical catalysts, chemists have devised sequential
Direct, enantioselective α-alkylation of aldehydes using simple olefins.
Capacci AG, et al.
Nature Chemistry, 9(11), 1073-1073 (2017)
Jack A Terrett et al.
Journal of the American Chemical Society, 136(19), 6858-6861 (2014-04-24)
Direct β-alkylation of saturated aldehydes has been accomplished by synergistically combining photoredox catalysis and organocatalysis. Photon-induced enamine oxidation provides an activated β-enaminyl radical intermediate, which readily combines with a wide range of Michael acceptors to produce β-alkyl aldehydes in a
Direct β -alkylation of aldehydes via photoredox organocatalysis.
Terrett JA, et al.
Journal of the American Chemical Society, 136(19), 6858-6861 (2014)
Andrew G Capacci et al.
Nature chemistry, 9(11), 1073-1077 (2017-10-25)
Although the α-alkylation of ketones has already been established, the analogous reaction using aldehyde substrates has proven surprisingly elusive. Despite the structural similarities between the two classes of compounds, the sensitivity and unique reactivity of the aldehyde functionality has typically
Active Filters
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務![(Ir[dF(CF3)ppy]2(dtbpy))PF6](/deepweb/assets/sigmaaldrich/product/structures/982/913/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09/640/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09.png)
![[Ir(dtbbpy)(ppy)2]PF6](/deepweb/assets/sigmaaldrich/product/structures/158/329/2544d673-d267-4aa1-8f46-2652aad4bfa0/640/2544d673-d267-4aa1-8f46-2652aad4bfa0.png)
![[铱 (dF (Me) ppy)] 2 (dtbbpy) ] PF 6](/deepweb/assets/sigmaaldrich/product/structures/150/099/7c2dfa31-39f4-4cca-aee5-86d4a89fea78/640/7c2dfa31-39f4-4cca-aee5-86d4a89fea78.png)
![[Ir{dFCF3ppy}2(bpy)]PF6](/deepweb/assets/sigmaaldrich/product/structures/180/924/79119ac4-7d62-429d-b23d-a14c012c6050/640/79119ac4-7d62-429d-b23d-a14c012c6050.png)
![[Ir(dFppy)2(dtbbpy)]PF6](/deepweb/assets/sigmaaldrich/product/structures/258/715/c8fe85d5-be71-4ff1-849b-a20766636770/640/c8fe85d5-be71-4ff1-849b-a20766636770.png)
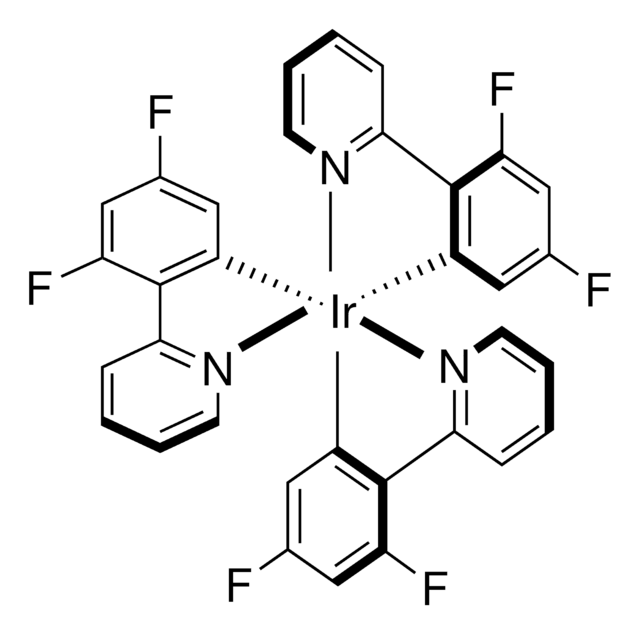

![[Ir(dFCF3ppy)2-(5,5’-dCF3bpy)]PF6 ≥95%](/deepweb/assets/sigmaaldrich/product/structures/422/901/e00f3148-fb86-4f94-9e79-21d064c3f327/640/e00f3148-fb86-4f94-9e79-21d064c3f327.png)
![Ir[dFFppy]2-(4,4′-dCF3bpy)PF6 ≥95%](/deepweb/assets/sigmaaldrich/product/structures/816/772/b116c17c-e6b2-4c95-be64-45a5a851d823/640/b116c17c-e6b2-4c95-be64-45a5a851d823.png)
![三[2-苯基吡啶-C2,N]铱(III) 97%](/deepweb/assets/sigmaaldrich/product/structures/167/234/658d0b76-d31d-4fd5-8041-e04e207227c9/640/658d0b76-d31d-4fd5-8041-e04e207227c9.png)
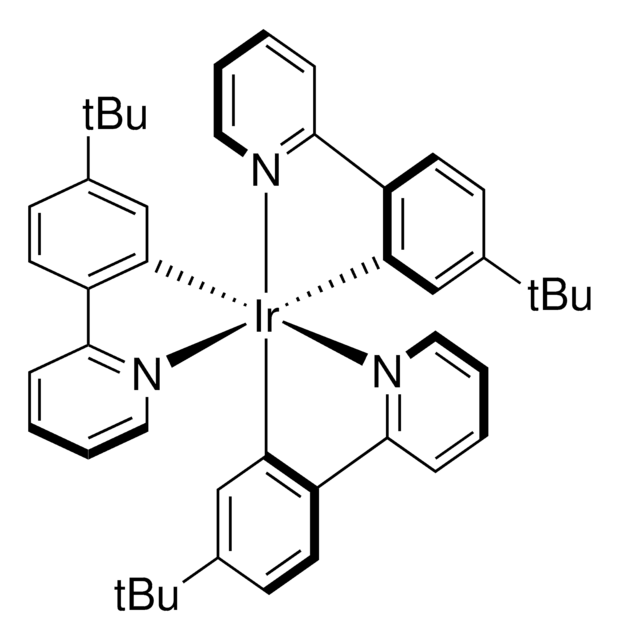
2 ≥95%](/deepweb/assets/sigmaaldrich/product/structures/190/371/c5efe61d-383f-4364-90c6-1912d88674f3/640/c5efe61d-383f-4364-90c6-1912d88674f3.png)

![铱 [ p -F (t-Bu)-ppy] 3](/deepweb/assets/sigmaaldrich/product/structures/189/186/7badaac3-82af-4109-aab5-dea3a3aa916d/640/7badaac3-82af-4109-aab5-dea3a3aa916d.png)