推薦產品
形狀
powder
反應適用性
reaction type: Photocatalysis
reagent type: catalyst
應用
This photocatalyst facilitates a variety of transformations using visible light, including the benzylation of aldehydes with alcohols.
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
其他說明
Spin-Center Shift-Enabled Direct Enantioselective α-Benzylation of Aldehydes with Alcohols
Photocatalytic Generation of 2-Azolyl Radicals: Intermediates for the Azolylation of Arenes and Heteroarenes via C–H Functionalization
Visible-Light Photocatalytic Decarboxylation of α,β-Unsaturated Carboxylic Acids: Facile Access to Stereoselective Difluoromethylated Styrenes in Batch and Flow
Photocatalytic Generation of 2-Azolyl Radicals: Intermediates for the Azolylation of Arenes and Heteroarenes via C–H Functionalization
Visible-Light Photocatalytic Decarboxylation of α,β-Unsaturated Carboxylic Acids: Facile Access to Stereoselective Difluoromethylated Styrenes in Batch and Flow
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
Xiao-Jing Wei et al.
ACS catalysis, 7(10), 7136-7140 (2017-11-08)
The development of synthetic methodologies which provide access to both stereoisomers of α,β-disubstituted olefins is a challenging undertaking. Herein, we describe the development of an operationally simple and stereoselective synthesis of difluoromethylated styrenes via a visible-light photocatalytic decarboxylation strategy using
Eric D Nacsa et al.
Journal of the American Chemical Society, 140(9), 3322-3330 (2018-02-06)
Nature routinely engages alcohols as leaving groups, as DNA biosynthesis relies on the removal of water from ribonucleoside diphosphates by a radical-mediated "spin-center shift" (SCS) mechanism. Alcohols, however, remain underused as alkylating agents in synthetic chemistry due to their low
Amandeep Arora et al.
Organic letters, 18(16), 3996-3999 (2016-08-06)
The 2-azolyl radical, generated from 2-bromoazoles via photocatalysis, is a powerful intermediate for the intermolecular arylation of unmodified (hetero)arenes. The reaction is characterized by mild conditions, operational simplicity, tolerance toward functional and sterically demanding groups, broad scope, and anti-Minisci selectivity.
Facile synthesis and complete characterization of homoleptic and heteroleptic cyclometalated Iridium(III) complexes for photocatalysis
Singh A, et al.
Journal of Organometallic Chemistry, 776, 51-59 (2015)
相關內容
Organofluorine chemistry is an essential part of drug discovery programs as well as agrochemical programs and even plays a major role in materials chemistry. Despite the undeniable importance of fluorinated organic molecules, our ability to synthesize these substrates is lacking - though arguably it is better than that of Nature. Consequently, methods that allow facile access to fluorinated molecules are important especially when they provide unique access to fluorinated chemical space.
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務![(Ir[dF(CF3)ppy]2(dtbpy))PF6](/deepweb/assets/sigmaaldrich/product/structures/982/913/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09/640/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09.png)
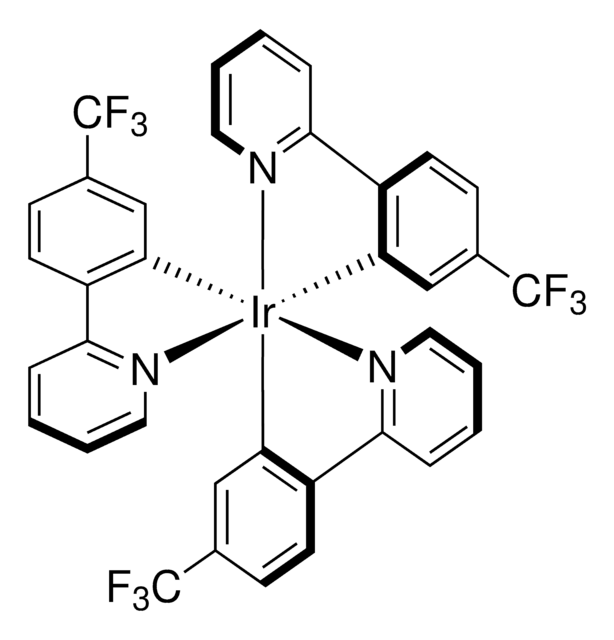
![三[2-苯基吡啶-C2,N]铱(III) 97%](/deepweb/assets/sigmaaldrich/product/structures/167/234/658d0b76-d31d-4fd5-8041-e04e207227c9/640/658d0b76-d31d-4fd5-8041-e04e207227c9.png)
![[Ir(dtbbpy)(ppy)2]PF6](/deepweb/assets/sigmaaldrich/product/structures/158/329/2544d673-d267-4aa1-8f46-2652aad4bfa0/640/2544d673-d267-4aa1-8f46-2652aad4bfa0.png)


![Ir[dF(t-Bu)-ppy]3](/deepweb/assets/sigmaaldrich/product/structures/254/294/d0fb19e5-05b2-4c1b-990b-a99fa60b3e73/640/d0fb19e5-05b2-4c1b-990b-a99fa60b3e73.png)
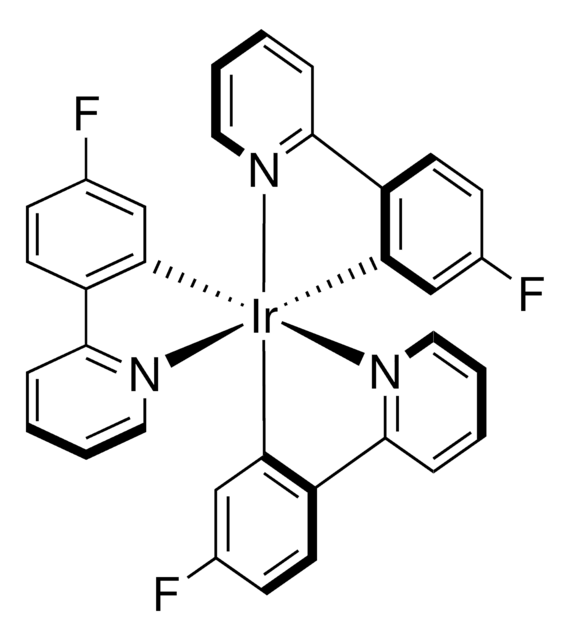
![[铱 (dF (Me) ppy)] 2 (dtbbpy) ] PF 6](/deepweb/assets/sigmaaldrich/product/structures/150/099/7c2dfa31-39f4-4cca-aee5-86d4a89fea78/640/7c2dfa31-39f4-4cca-aee5-86d4a89fea78.png)
