H1015
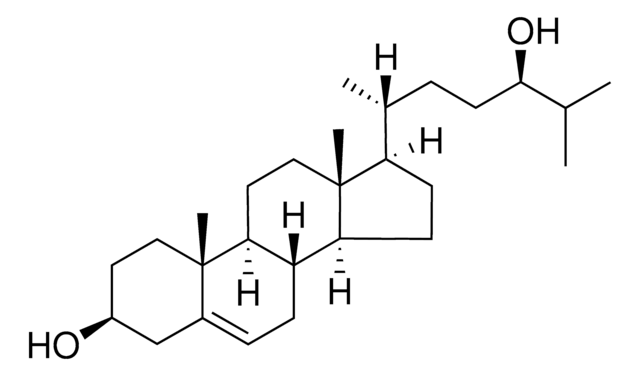
25-Hydroxycholesterol
≥98%
Synonim(y):
5-Cholestene-3β,25-diol
About This Item
Polecane produkty
pochodzenie biologiczne
synthetic (organic)
Próba
≥98%
Postać
powder
Warunki transportu
ambient
temp. przechowywania
room temp
ciąg SMILES
[H][C@@]12[C@]([C@](CC[C@H](O)C3)(C)C3=CC2)([H])CC[C@@]4(C)[C@@]1([H])CC[C@]4([H])[C@]([H])(C)CCCC(C)(O)C
InChI
1S/C27H46O2/c1-18(7-6-14-25(2,3)29)22-10-11-23-21-9-8-19-17-20(28)12-15-26(19,4)24(21)13-16-27(22,23)5/h8,18,20-24,28-29H,6-7,9-17H2,1-5H3/t18-,20+,21+,22-,23+,24+,26+,27-/m1/s1
Klucz InChI
INBGSXNNRGWLJU-ZHHJOTBYSA-N
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Zastosowanie
Działania biochem./fizjol.
Uwaga dotycząca przygotowania
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Certyfikaty analizy (CoA)
Poszukaj Certyfikaty analizy (CoA), wpisując numer partii/serii produktów. Numery serii i partii można znaleźć na etykiecie produktu po słowach „seria” lub „partia”.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Produkty
Cholesterol synthesis regulation by dietary levels, LDL receptors control lipid-rich LDL particle transport in cells.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej