495604
Okadaic acid
from Prorocentrum sp., ≥95% (HPLC), solid (glassy), protein phosphatase 1 inhibitor, Calbiochem
Sinonimo/i:
Okadaic Acid, Prorocentrum sp., OA
Scegli un formato
Scegli un formato
About This Item
Prodotti consigliati
Nome del prodotto
Okadaic Acid, Prorocentrum sp., Okadaic Acid, CAS 78111-17-8, is a highly potent inhibitor of protein phosphatase 1 (IC₅₀ = 10-15 nM) and 2A (IC₅₀ = 0.1 nM).
Livello qualitativo
Saggio
≥95% (HPLC)
Stato
solid (glassy)
Produttore/marchio commerciale
Calbiochem®
Condizioni di stoccaggio
OK to freeze
protect from light
Colore
colorless
Solubilità
DMF: soluble
chloroform: soluble
ethanol: soluble
methanol: soluble
Condizioni di spedizione
ambient
Temperatura di conservazione
−20°C
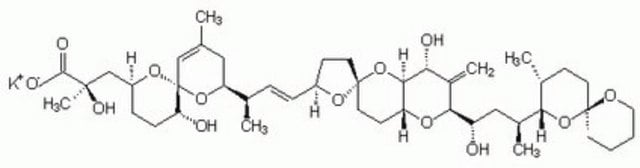
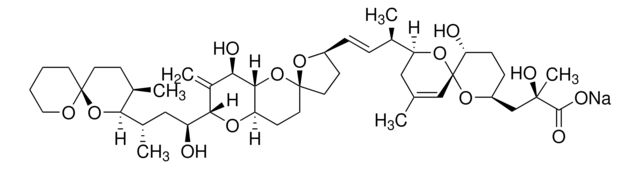
Stringa SMILE
O1[C@]7(O[C@@H](CC[C@H]7O)C[C@](O)(C)C(=O)O)C=C(C[C@H]1[C@H](C)\C=C\[C@@H]2O[C@@]3(OC4[C@H](O[C@@H](C(=C)[C@H]4O)[C@@H](O)C[C@@H](C5O[C@]6(OCCCC6)CC[C@H]5C)C)CC3)CC2)C
InChI
1S/C44H68O13/c1-25-21-34(55-44(23-25)35(46)12-11-31(54-44)24-41(6,50)40(48)49)26(2)9-10-30-14-18-43(53-30)19-15-33-39(57-43)36(47)29(5)38(52-33)32(45)22-28(4)37-27(3)13-17-42(56-37)16-7-8-20-51-42/h9-10,23,26-28,30-39,45-47,50H,5,7-8,11-22,24H2,1-4,6H3,(H,48,49)/b10-9+/t26-,27-,28+,30+,31+,32+,33-,34+,35-,36-,37?,38+,39?,41-,42+,43-,44-/m1/s1
QNDVLZJODHBUFM-AAWJMCDUSA-N
Descrizione generale
Azioni biochim/fisiol
Protein phosphatase
Confezionamento
Attenzione
Ricostituzione
Altre note
Kiguchi, K., et al. 1994. Cell Growth Differentiation5, 995.
Ohaka, Y., et al. 1993. Biochem. Biophys. Res. Commun. 197, 916.
Gopalakrishna, R., et al. 1992. Biochem. Biophys. Res. Commun. 189, 950.
Kreienbuhl, P., et al. 1992. Blood80, 2911.
Nomura, M., et al. 1992. Biochemistry31, 11915.
Song, Q., et al. 1992. J. Cell Physiol.153, 550.
Tada, Y., et al. 1992. Immunopharmacol.24, 17.
Cohen, P., et al. 1990. Trends Biochem. Sci.15, 98.
Cohen, P. 1989. Annu. Rev. Biochem.58, 453.
Cohen, P., and Cohen, P.T. 1989. J. Biol. Chem.264, 21435.
Haystead, T.A., et al. 1989. Nature337, 78.
Note legali
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Skin Irrit. 2
Codice della classe di stoccaggio
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Filtri attivi
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.