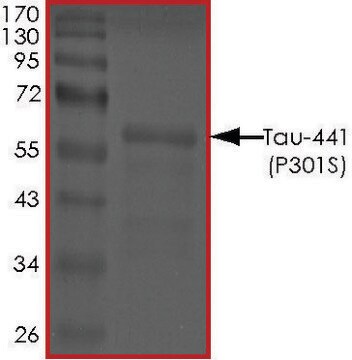
Összes fotó(1)
Fontos dokumentumok
T0326
Tau-412 human
recombinant, expressed in E. coli, ≥90% (SDS-PAGE), lyophilized powder
Bejelentkezésa Szervezeti és Szerződéses árazás megtekintéséhez
Összes fotó(1)
About This Item
Javasolt termékek
biológiai forrás
human
Minőségi szint
rekombináns
expressed in E. coli
Teszt
≥90% (SDS-PAGE)
Forma
lyophilized powder
molekulatömeg
42.9 kDa
UniProt elérési szám
alkalmazás(ok)
cell analysis
kiszállítva
dry ice
tárolási hőmérséklet
−70°C
Géninformáció
human ... MAPT(4137)
Általános leírás
Tau-412 human, (1N4R) recombinant, is one of the isoforms of Tau and lacks a projection domain region. The isoforms of tau are generated by alternate splicing. The N terminal region of tau is acidic and is separated from the C-terminal tubulin-binding region by a central proline-rich region. The isoforms differ in amino-terminal N1 and N2 regions as well as binding repeats at the tubulin region. Tau belongs to the family of neuronal microtubule-associated proteins.
Biokémiai/fiziológiai hatások
Tau promotes assembly and stabilizes neuronal microtubules under normal physiological conditions. Under pathological conditions Tau can also undergo modifications such as hyperphosphorylation, which can result in the generation of aberrant aggregates such as found in neurofibrillary tangles in Alzheimer′s disease. Tau-412 human is purified without any acid treatment and is suitable for promoting microtubule assembly, and for hyperphosphorylation-induced self-assembly into filaments.
Isoform of Tau, variant 1N4R, having 4 microtubule binding repeats (R) and one amino terminal insert (N).
Feloldás
Lyophilized from MES, pH 6.8, containing NaCl and EGTA. When reconstituted in water to a protein concentration of 1 mg/mL, the resulting buffer will have ~50 mM MES, pH 6.8, 100 mM NaCl, and 0.5 mM EGTA.
Tárolási osztály kódja
11 - Combustible Solids
WGK
WGK 1
Lobbanási pont (F)
Not applicable
Lobbanási pont (C)
Not applicable
Egyéni védőeszköz
Eyeshields, Gloves, type N95 (US)
Válasszon a legfrissebb verziók közül:
Már rendelkezik ezzel a termékkel?
Az Ön által nemrégiben megvásárolt termékekre vonatkozó dokumentumokat a Dokumentumtárban találja.
Abolfazl Jangholi et al.
Archives of biochemistry and biophysics, 609, 1-19 (2016-09-18)
In many neurodegenerative diseases, formation of protein fibrillar aggregates has been observed as a major pathological change. Neurofibrillary tangles, mainly composed of fibrils formed by the microtubule-associated protein; Tau, are a hallmark of a group of neurodegenerative diseases such as
Simon Moussaud et al.
Molecular neurodegeneration, 9, 43-43 (2014-10-30)
The accumulation of α-synuclein aggregates is the hallmark of Parkinson's disease, and more generally of synucleinopathies. The accumulation of tau aggregates however is classically found in the brains of patients with dementia, and this type of neuropathological feature specifically defines
Jesus Avila et al.
Physiological reviews, 84(2), 361-384 (2004-03-27)
The morphology of a neuron is determined by its cytoskeletal scaffolding. Thus proteins that associate with the principal cytoskeletal components such as the microtubules have a strong influence on both the morphology and physiology of neurons. Tau is a microtubule-associated
A Himmler et al.
Molecular and cellular biology, 9(4), 1381-1388 (1989-04-01)
Tau proteins consist of a family of proteins, heterogeneous in size, which associate with microtubules in vivo and are induced during neurite outgrowth. In humans, tau is one of the major components of the pathognomonic neurofibrillary tangles in Alzheimer's disease
M Goedert et al.
Neuron, 3(4), 519-526 (1989-10-01)
We have determined the sequences of isoforms of human tau protein, which differ from previously reported forms by insertions of 29 or 58 amino acids in the amino-terminal region. Complementary DNA cloning shows that the insertions occur in combination with
Tudóscsoportunk valamennyi kutatási területen rendelkezik tapasztalattal, beleértve az élettudományt, az anyagtudományt, a kémiai szintézist, a kromatográfiát, az analitikát és még sok más területet.
Lépjen kapcsolatba a szaktanácsadással