Fontos dokumentumok
M7773
Monoclonal Anti-Myoglobin antibody produced in mouse
clone MG-1, ascites fluid
Szinonimák:
Anti-PVALB
About This Item
IHC (p)
indirect ELISA: 1:10,000
Javasolt termékek
biológiai forrás
mouse
Minőségi szint
konjugátum
unconjugated
antitest forma
ascites fluid
antitest terméktípus
primary antibodies
klón
MG-1, monoclonal
tartalmaz
15 mM sodium azide
faj reaktivitás
human
technika/technikák
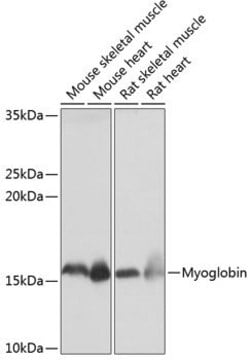
immunohistochemistry (formalin-fixed, paraffin-embedded sections): 1:400 using human skeletal muscle tissue
indirect ELISA: 1:10,000
izotípus
IgG1
UniProt elérési szám
kiszállítva
dry ice
tárolási hőmérséklet
−20°C
célzott transzláció utáni módosítás
unmodified
Géninformáció
human ... MB(4151)
Related Categories
Általános leírás
Immunogén
Alkalmazás
Biokémiai/fiziológiai hatások
Jogi nyilatkozat
Nem találja a megfelelő terméket?
Próbálja ki a Termékválasztó eszköz. eszközt
Tárolási osztály kódja
10 - Combustible liquids
WGK
nwg
Lobbanási pont (F)
Not applicable
Lobbanási pont (C)
Not applicable
Válasszon a legfrissebb verziók közül:
Analitikai tanúsítványok (COA)
Nem találja a megfelelő verziót?
Ha egy adott verzióra van szüksége, a tétel- vagy cikkszám alapján rákereshet egy adott tanúsítványra.
Már rendelkezik ezzel a termékkel?
Az Ön által nemrégiben megvásárolt termékekre vonatkozó dokumentumokat a Dokumentumtárban találja.
Tudóscsoportunk valamennyi kutatási területen rendelkezik tapasztalattal, beleértve az élettudományt, az anyagtudományt, a kémiai szintézist, a kromatográfiát, az analitikát és még sok más területet.
Lépjen kapcsolatba a szaktanácsadással