428426
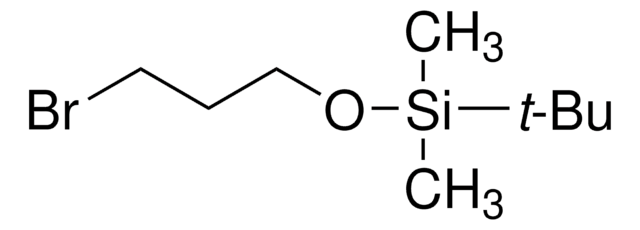
(2-Bromoethoxy)-tert-butyldimethylsilane
99%
Přihlásitk zobrazení cen stanovených pro organizaci a smluvních cen
About This Item
Lineární vzorec:
BrCH2CH2OSi(CH3)2C(CH3)3
Číslo CAS:
Molekulová hmotnost:
239.23
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Doporučené produkty
assay
99%
form
liquid
contains
Na2CO3 as stabilizer
refractive index
n20/D 1.444 (lit.)
bp
70-75 °C/2.5 mmHg (lit.)
density
1.115 g/mL at 25 °C (lit.)
functional group
bromo
storage temp.
2-8°C
SMILES string
CC(C)(C)[Si](C)(C)OCCBr
InChI
1S/C8H19BrOSi/c1-8(2,3)11(4,5)10-7-6-9/h6-7H2,1-5H3
InChI key
JBKINHFZTVLNEM-UHFFFAOYSA-N
General description
(2-Bromoethoxy)-tert-butyldimethylsilane is a silane derivative.
Application
(2-Bromoethoxy)-tert-butyldimethylsilane was used in the synthesis of 4-(3-hydroxypropyl)-4′-methyl-2,2′-bipyridine.
It may be used as a reagent for the selective N-alkylation of 5-piperazin-1-yl-1H-indole and (1H-indol-2-yl)-piperazin-1-yl-methanone and also in the synthesis of 2-[3-[(3,4,5-trimethoxyphenyl)thio]-1H-indol-5-yloxy]ethanol.
It may be used as a reagent for the selective N-alkylation of 5-piperazin-1-yl-1H-indole and (1H-indol-2-yl)-piperazin-1-yl-methanone and also in the synthesis of 2-[3-[(3,4,5-trimethoxyphenyl)thio]-1H-indol-5-yloxy]ethanol.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
163.4 °F - closed cup
flash_point_c
73 °C - closed cup
Vyberte jednu z posledních verzí:
Již tento produkt vlastníte?
Dokumenty související s produkty, které jste v minulosti zakoupili, byly za účelem usnadnění shromážděny ve vaší Knihovně dokumentů.
Zákazníci si také prohlíželi
Tetrahedron Letters, 47, 69-69 (2006)
Hansen, Joshua; et al.
Tetrahedron Letters, 47(1), 69-72 (2005)
Cyril Bressy et al.
Journal of the American Chemical Society, 127(38), 13148-13149 (2005-09-22)
A norbornene-mediated palladium-catalyzed tandem alkylation/C-H functionalization sequence is described, in which an alkyl-aryl bond and a heteroaryl-aryl bond are formed in one pot. A variety of highly substituted six- and seven-membered ring annulated indoles were synthesized in good yields from
Arylthioindole inhibitors of tubulin polymerization. 3. Biological evaluation, structure-activity relationships and molecular modeling studies.
La Regina G, et al.
Journal of Medicinal Chemistry, 50(12), 2865-2874 (2007)
Mark Johnson et al.
Journal of medicinal chemistry, 55(12), 5826-5840 (2012-05-31)
In our effort to develop multifunctional drugs against Parkinson's disease, a structure-activity-relationship study was carried out based on our hybrid molecular template targeting D2/D3 receptors. Competitive binding with [(3)H]spiroperidol was used to evaluate affinity (K(i)) of test compounds. Functional activity
Náš tým vědeckých pracovníků má zkušenosti ve všech oblastech výzkumu, včetně přírodních věd, materiálových věd, chemické syntézy, chromatografie, analytiky a mnoha dalších..
Obraťte se na technický servis.