HPA020376
Anti-PSPH antibody produced in rabbit

Prestige Antibodies® Powered by Atlas Antibodies, affinity isolated antibody, buffered aqueous glycerol solution
Sinônimo(s):
Anti-L-3-phosphoserine phosphatase, Anti-O-phosphoserine phosphohydrolase, Anti-PSP, Anti-PSPase, Anti-Phosphoserine phosphatase
Selecione um tamanho
R$ 4.140,00
Previsão de entrega em07 de maio de 2025
Selecione um tamanho
About This Item
R$ 4.140,00
Previsão de entrega em07 de maio de 2025
Produtos recomendados
fonte biológica
rabbit
Nível de qualidade
conjugado
unconjugated
forma do anticorpo
affinity isolated antibody
tipo de produto de anticorpo
primary antibodies
clone
polyclonal
linha de produto
Prestige Antibodies® Powered by Atlas Antibodies
Formulário
buffered aqueous glycerol solution
reatividade de espécies
human
validação aprimorada
orthogonal RNAseq
Learn more about Antibody Enhanced Validation
técnica(s)
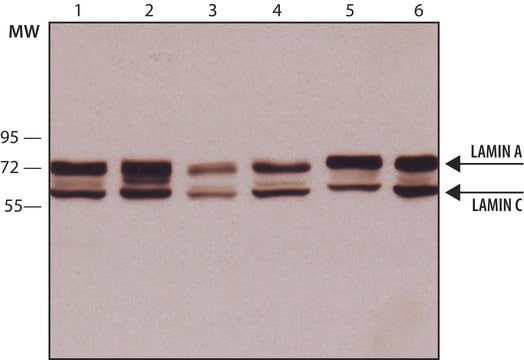
immunoblotting: 0.04-0.4 μg/mL
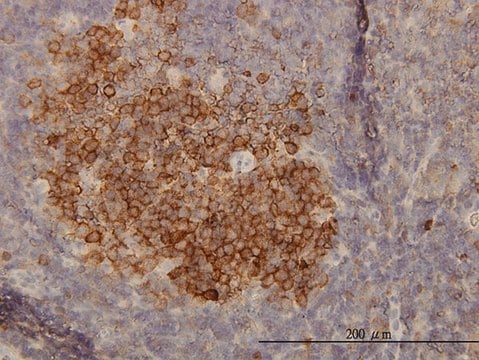
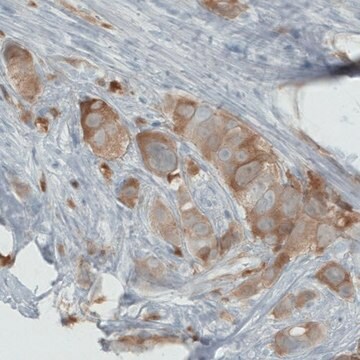
immunohistochemistry: 1:200-1:500
sequência de imunogênio
AVCFDVDSTVIREEGIDELAKICGVEDAVSEMTRRAMGGAVPFKAALTERLALIQPSREQVQRLIAEQPPHLTPGIRELVSRLQERNVQVFLISG
nº de adesão UniProt
Condições de expedição
wet ice
temperatura de armazenamento
−20°C
modificação pós-traducional do alvo
unmodified
Informações sobre genes
human ... PSPH(5723)
Descrição geral
Imunogênio
Aplicação
Western Blotting (1 paper)
Ações bioquímicas/fisiológicas
Características e benefícios
Every Prestige Antibody is tested in the following ways:
- IHC tissue array of 44 normal human tissues and 20 of the most common cancer type tissues.
- Protein array of 364 human recombinant protein fragments.
Ligação
forma física
Informações legais
Exoneração de responsabilidade
Não está encontrando o produto certo?
Experimente o nosso Ferramenta de seleção de produtos.
Código de classe de armazenamento
10 - Combustible liquids
Classe de risco de água (WGK)
WGK 1
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Escolha uma das versões mais recentes:
Certificados de análise (COA)
Não está vendo a versão correta?
Se precisar de uma versão específica, você pode procurar um certificado específico pelo número do lote ou da remessa.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Active Filters
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica