Products may be shipped at a different temperature than the recommended long-term storage temperature. If the product quality is sensitive to short-term exposure to conditions other than the recommended long-term storage, it will be shipped on wet or dry-ice. If the product quality is NOT affected by short-term exposure to conditions other than the recommended long-term storage, it will be shipped at ambient temperature. As shipping routes are configured for minimum transit times, shipping at ambient temperature helps control shipping costs for our customers. For more information, please refer to the Storage and Transport Conditions document: https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/marketing/global/documents/316/622/storage-transport-conditions-mk.pdf
03449
N-(3-dimetilaminopropil)-N′-etilcarbodiimida
≥99.0% (AT)
Sinônimo(s):
N-etil-N′-(3-dimetilaminopropil)carbodiimida, EDAC, EDC, WSC
Selecione um tamanho
Selecione um tamanho
About This Item
Produtos recomendados
Ensaio
≥99.0% (AT)
Formulário
powder
técnica(s)
bioconjugation: suitable
pf
110-115 °C (lit.)
112-116 °C
solubilidade
H2O: soluble 0.2 g/L
temperatura de armazenamento
−20°C
cadeia de caracteres SMILES
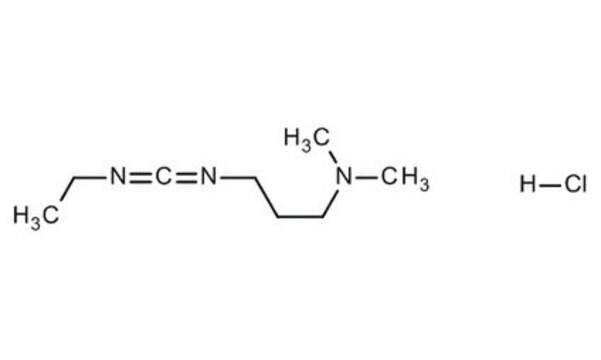
Cl.CCN=C=NCCCN(C)C
Cl.CCN=C=NCCCN(C)C
InChI
1S/C8H17N3.ClH/c1-4-9-8-10-6-5-7-11(2)3;/h4-7H2,1-3H3;1H
chave InChI
FPQQSJJWHUJYPU-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
Furthermore, EDAC HCl serves as a biomolecule bridge, acting as a crosslinker that connects amine-reactive NHS-esters of biomolecules to carboxyl groups. This technique proves invaluable in protein conjugation, enabling the creation of hybrid molecules with novel properties and functions. The underlying mechanism involves EDAC HCl′s reaction with a carboxyl group, forming an unstable intermediate that actively seeks an amine partner. The delicate balance of this reaction underscores the importance of optimizing conditions for efficient conjugation. The assistance of N-hydroxysuccinimide (NHS) further enhances EDAC HCl′s capabilities by stabilizing the intermediate and enabling two-step conjugation procedures. This additional feature provides greater flexibility and control, particularly when dealing with complex biomolecules.
Aplicação
Ações bioquímicas/fisiológicas
Características e benefícios
Outras notas
produto comparável
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 3 Dermal - Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Skin Irrit. 2 - Skin Sens. 1 - STOT RE 2 Oral
Órgãos-alvo
Stomach,large intestine,lymph node
Código de classe de armazenamento
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Artigos
Professor Aran discusses engineering graphene-based materials through careful functionalization, enabling diverse applications.
-
How is shipping temperature determined? And how is it related to the product storage temperature?
1 answer-
Helpful?
-
-
How can I determine the shelf life / expiration / retest date of this product?
1 answer-
If this product has an expiration or retest date, it will be shown on the Certificate of Analysis (COA, CofA). If there is no retest or expiration date listed on the product's COA, we do not have suitable stability data to determine a shelf life. For these products, the only date on the COA will be the release date; a retest, expiration, or use-by-date will not be displayed.
For all products, we recommend handling per defined conditions as printed in our product literature and website product descriptions. We recommend that products should be routinely inspected by customers to ensure they perform as expected.
For products without retest or expiration dates, our standard warranty of 1 year from the date of shipment is applicable.
For more information, please refer to the Product Dating Information document: https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/marketing/global/documents/449/386/product-dating-information-mk.pdfHelpful?
-
Active Filters
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica








![1-[3-(Dimethylamino)propyl]-3-ethylcarbodiimide methiodide](/deepweb/assets/sigmaaldrich/product/structures/414/134/4eb9c126-d7f9-4e12-9e3a-95cb077824fd/640/4eb9c126-d7f9-4e12-9e3a-95cb077824fd.png)



