03450
N-(3-dimetilaminopropil)-N′-etilcarbodiimida
purum, ≥98.0% (AT)
Sinônimo(s):
N-etil-N′-(3-dimetilaminopropil)carbodiimida, EDAC, EDC, WSC
Selecione um tamanho
Selecione um tamanho
About This Item
Produtos recomendados
grau
purum
Nível de qualidade
Ensaio
≥98.0% (AT)
Formulário
powder
pf
110-115 °C (lit.)
110-115 °C
solubilidade
H2O: soluble 1 gm/10 ml, clear to very slightly hazy, colorless to very faintly yellow
aplicação(ões)
microbiology
temperatura de armazenamento
−20°C
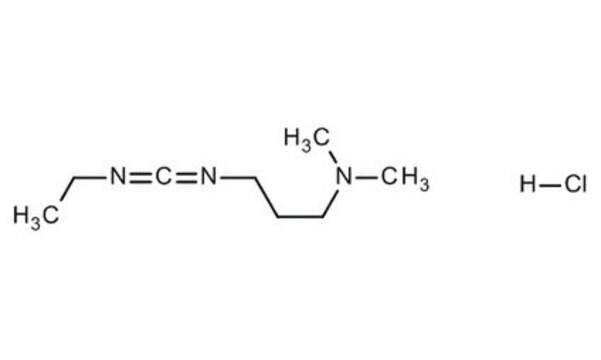
cadeia de caracteres SMILES
Cl.CCN=C=NCCCN(C)C
InChI
1S/C8H17N3.ClH/c1-4-9-8-10-6-5-7-11(2)3;/h4-7H2,1-3H3;1H
chave InChI
FPQQSJJWHUJYPU-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
The adaptability of EDAC HCl encompasses nucleic acid modification, permitting the selective labeling of DNA and RNA through their 5′ phosphate groups. This capacity contributes significantly to the visualization, tracking, and analytical aspects of these fundamental molecules, thereby advancing nucleic acid research. Furthermore, EDAC HCl functions as a biomolecule bridge, acting as a crosslinker connecting amine-reactive NHS-esters of biomolecules to carboxyl groups.
This feature proves invaluable in protein conjugation, facilitating the development of hybrid molecules with distinct properties and functions. The underlying reaction mechanism involves EDAC HCl′s interaction with a carboxyl group, forming an unstable intermediate actively seeking an amine partner. The delicate equilibrium of this reaction underscores the necessity for optimizing conditions to ensure efficient conjugation. The assistance of N-hydroxysuccinimide (NHS) enhances the capabilities of EDAC HCl by stabilizing the intermediate and enabling two-step conjugation procedures, affording greater flexibility and control, particularly in the manipulation of complex biomolecular structures.
Aplicação
Ações bioquímicas/fisiológicas
Características e benefícios
Outras notas
produto comparável
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 3 Dermal - Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Skin Irrit. 2 - Skin Sens. 1 - STOT RE 2 Oral
Órgãos-alvo
Stomach,large intestine,lymph node
Código de classe de armazenamento
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Active Filters
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica