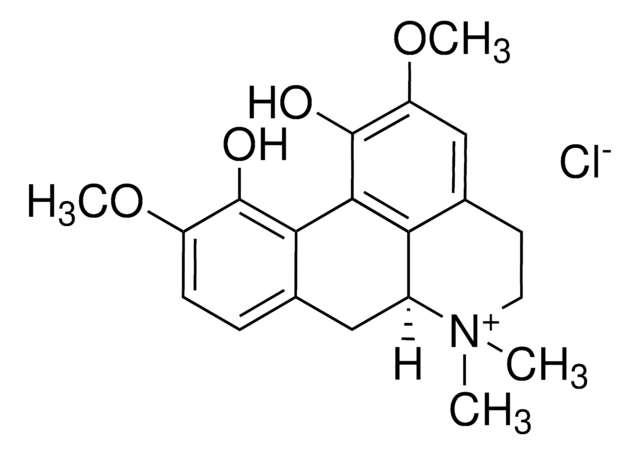
5.33978
ACC Inhibitor IV, CP-640186
Synonym(e):
ACC Inhibitor IV, CP-640186, 9-Anthryl((3 R)-3-(4-morpholinylcarbonyl)-1,4ʹ-bipiperidin-1ʹ-yl)methanone, HCl, Acetyl-CoA Carboxylase Inhibitor IV, CP-640186, HCl
About This Item
Empfohlene Produkte
Assay
≥98% (HPLC)
Qualitätsniveau
Form
solid
Hersteller/Markenname
Calbiochem®
Lagerbedingungen
OK to freeze
desiccated (hygroscopic)
protect from light
Farbe
light orange
Löslichkeit
DMSO: 50 mg/mL
H2O: 50 mg/mL
Lagertemp.
2-8°C
Allgemeine Beschreibung
Please note that the molecular weight for this compound is batch-specific due to variable water content. Please refer to the vial label or the certificate of analysis for the batch-specific molecular weight. The molecular weight provided represents the baseline molecular weight without water.
Biochem./physiol. Wirkung
Acetyl-CoA carboxylase (ACC)
Warnhinweis
Physikalische Form
Rekonstituierung
Sonstige Hinweise
Gu, Y.G., et al. 2006. J. Med. Chem.49, 3770.
Zhang, H., et al. 2004. Structure12, 1683.
Harwood, H.J., et al. 2003. J. Biol. Chem.278, 37099.
Rechtliche Hinweise
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.