推荐产品
表单
powder
反应适用性
core: iridium
reagent type: catalyst
reaction type: Photocatalysis
光触媒活化
465 nm
SMILES字符串
F[P-](F)(F)(F)(F)F.CC1=CC([Ir+]([N]2=C3C=C(C)C=C2)(C4=C5C=CC(C)=C4)([N]6=C7C=C(C(C)(C)C)C=C6)([N]8=C7C=C(C(C)(C)C)C=C8)[N]9=C5C=C(C)C=C9)=C3C=C1
应用
(Ir[Me(Me)ppy]2(dtbpy))PF6 or Ir(dmppy)2(dtbbpy)PF6 is an iridium photoredox catalyst that facilitates a variety of transformations using visible light, including the α- and β-alkylation of aldehydes.[1][2]
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
相关产品
产品编号
说明
价格
储存分类代码
11 - Combustible Solids
WGK
WGK 3
闪点(°F)
Not applicable
闪点(°C)
Not applicable
其他客户在看
Direct β -alkylation of aldehydes via photoredox organocatalysis.
Terrett JA, et al.
Journal of the American Chemical Society, 136(19), 6858-6861 (2014)
Direct, enantioselective α-alkylation of aldehydes using simple olefins.
Capacci AG, et al.
Nature Chemistry, 9(11), 1073-1073 (2017)
Jack A Terrett et al.
Journal of the American Chemical Society, 136(19), 6858-6861 (2014-04-24)
Direct β-alkylation of saturated aldehydes has been accomplished by synergistically combining photoredox catalysis and organocatalysis. Photon-induced enamine oxidation provides an activated β-enaminyl radical intermediate, which readily combines with a wide range of Michael acceptors to produce β-alkyl aldehydes in a
Zachary C Litman et al.
Nature, 560(7718), 355-359 (2018-08-17)
Living organisms rely on simultaneous reactions catalysed by mutually compatible and selective enzymes to synthesize complex natural products and other metabolites. To combine the advantages of these biological systems with the reactivity of artificial chemical catalysts, chemists have devised sequential
Andrew G Capacci et al.
Nature chemistry, 9(11), 1073-1077 (2017-10-25)
Although the α-alkylation of ketones has already been established, the analogous reaction using aldehyde substrates has proven surprisingly elusive. Despite the structural similarities between the two classes of compounds, the sensitivity and unique reactivity of the aldehyde functionality has typically
Active Filters
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持![(Ir[dF(CF3)ppy]2(dtbpy))PF6](/deepweb/assets/sigmaaldrich/product/structures/982/913/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09/640/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09.png)
![[Ir(dtbbpy)(ppy)2]PF6](/deepweb/assets/sigmaaldrich/product/structures/158/329/2544d673-d267-4aa1-8f46-2652aad4bfa0/640/2544d673-d267-4aa1-8f46-2652aad4bfa0.png)
![[铱 (dF (Me) ppy)] 2 (dtbbpy) ] PF 6](/deepweb/assets/sigmaaldrich/product/structures/150/099/7c2dfa31-39f4-4cca-aee5-86d4a89fea78/640/7c2dfa31-39f4-4cca-aee5-86d4a89fea78.png)
![[Ir{dFCF3ppy}2(bpy)]PF6](/deepweb/assets/sigmaaldrich/product/structures/180/924/79119ac4-7d62-429d-b23d-a14c012c6050/640/79119ac4-7d62-429d-b23d-a14c012c6050.png)
![[Ir(dFppy)2(dtbbpy)]PF6](/deepweb/assets/sigmaaldrich/product/structures/258/715/c8fe85d5-be71-4ff1-849b-a20766636770/640/c8fe85d5-be71-4ff1-849b-a20766636770.png)
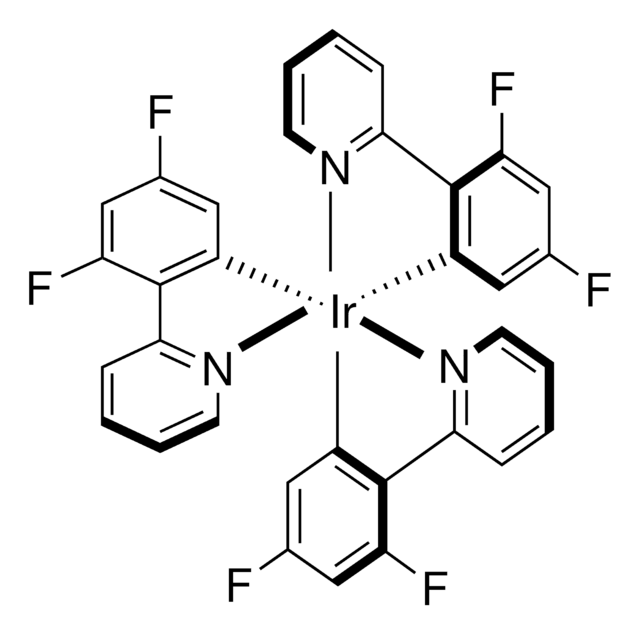

![[Ir(dFCF3ppy)2-(5,5’-dCF3bpy)]PF6 ≥95%](/deepweb/assets/sigmaaldrich/product/structures/422/901/e00f3148-fb86-4f94-9e79-21d064c3f327/640/e00f3148-fb86-4f94-9e79-21d064c3f327.png)
![Ir[dFFppy]2-(4,4′-dCF3bpy)PF6 ≥95%](/deepweb/assets/sigmaaldrich/product/structures/816/772/b116c17c-e6b2-4c95-be64-45a5a851d823/640/b116c17c-e6b2-4c95-be64-45a5a851d823.png)
![三[2-苯基吡啶-C2,N]铱(III) 97%](/deepweb/assets/sigmaaldrich/product/structures/167/234/658d0b76-d31d-4fd5-8041-e04e207227c9/640/658d0b76-d31d-4fd5-8041-e04e207227c9.png)
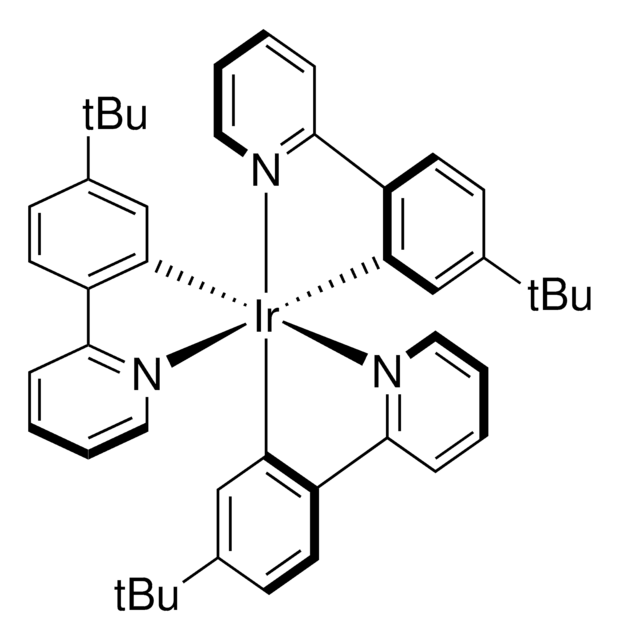
2 ≥95%](/deepweb/assets/sigmaaldrich/product/structures/190/371/c5efe61d-383f-4364-90c6-1912d88674f3/640/c5efe61d-383f-4364-90c6-1912d88674f3.png)

![铱 [ p -F (t-Bu)-ppy] 3](/deepweb/assets/sigmaaldrich/product/structures/189/186/7badaac3-82af-4109-aab5-dea3a3aa916d/640/7badaac3-82af-4109-aab5-dea3a3aa916d.png)