About This Item
Wzór empiryczny (zapis Hilla):
C217H341N71O74S9
Numer CAS:
Masa cząsteczkowa:
5417.05
Numer MDL:
Kod UNSPSC:
12352200
Polecane produkty
pochodzenie biologiczne
Echis carinatus
Próba
≥90% (HPLC)
Postać
powder
metody
flow cytometry: suitable
inhibition assay: suitable
temp. przechowywania
−20°C
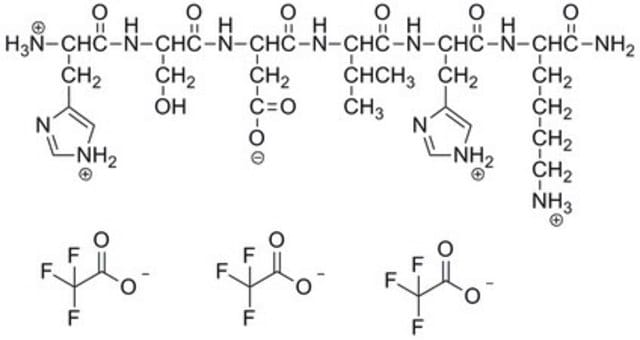
Amino Acid Sequence
Glu-Cys-Glu-Ser-Gly-Pro-Cys-Cys-Arg-Asn-Cys-Lys-Phe-Leu-Lys-Glu-Gly-Thr-Ile-Cys-Lys-Arg-Ala-Arg-Gly-Asp-Asp-Met-Asp-Asp-Tyr-Cys-Asn-Gly-Lys-Thr-Cys-Asp-Cys-Pro-Arg-Asn-Pro-His-Lys-Gly-Pro-Ala-Thr
Opis ogólny
Echistatin is a single chain 49 amino acid residue protein, which prevents the aggregation of platelets. It has an isoelectric point (pI) of 8.3 and a molecular weight of 5400. This peptide is present in the venom of Echis carinatus, which is a saw-scaled viper. It contains the arginine-glycine-aspartic (RGD) acid sequence, which is present in proteins that bind to glycoprotein IIb/IIIa complex. It shares the proline-arginine-asparagine-proline sequence with the Aα chain of human fibrinogen. This protein is a member of disintegrin family, which prevents cell adhesion. With regards to molecular weight, echistatins are the smallest member of disintegrin family, and contains four isoforms called, α1, α2, β and γ.
Zastosowanie
Echistatin has been used:
- as an inhibitor of integrin function to study the role of microfibril-associated glycoprotein-1 (Magp1) in the morphogenesis of vascular structures
- for the preparation of microbubbles targeted to αvβ3 integrins in tumor angiogenesis imaging
- as a conjugate to αvβ3 in flow cytometric binding studies
Działania biochem./fizjol.
Disintegrins represent a novel family of integrin β1 and β3 inhibitor proteins isolated from viper venoms. They are low molecular-weight, cysteine-rich peptides containing the Arg-Gly-Asp (RGD) sequence. They are the most potent known inhibitors of integrin function. Disintegrins interfere with cell adhesion to the extracellular matrix, including adhesion of melanoma cells and fibroblasts to fibronectin, and are potent inhibitors of platelet aggregation.
Echistatin is a disintegrin, which prevents the aggregation of platelets. They interact with and prevent the binding of fibrinogen to their receptors on the membrane of platelets. It also inhibits platelet aggregation mediated by epinephrine, thrombin, collagen, or platelet-activating factor. Studies in isolated osteoclasts show that this peptide inhibits bone resorption by osteoclasts, most probably by damaging adhesion structures.
This page may contain text that has been machine translated.
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Certyfikaty analizy (CoA)
Poszukaj Certyfikaty analizy (CoA), wpisując numer partii/serii produktów. Numery serii i partii można znaleźć na etykiecie produktu po słowach „seria” lub „partia”.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
M Gawaz et al.
Circulation, 96(6), 1809-1818 (1997-10-10)
Platelet interaction with endothelium plays an important role in the pathophysiology of coronary microcirculation. We assessed the role of the vitronectin receptor (integrin alpha(v)beta3) in platelet/endothelium adhesion. We investigated the effect on platelet/endothelium adhesion of plasma obtained from patients with
Z R Gan et al.
The Journal of biological chemistry, 263(36), 19827-19832 (1988-12-25)
A 49-residue protein, echistatin, which inhibits platelet aggregation, was purified from the venom of the saw-scaled viper Echis carinatus. The purification procedure included gel filtration on Sephadex G-50, cation-exchange chromatography on Mono S, and C18 reverse-phase high pressure liquid chromatography.
M Sato et al.
The Journal of cell biology, 111(4), 1713-1723 (1990-10-01)
The venom protein, s-echistatin, originally derived from the saw-scaled viper Echis carinatus, was found to be a potent inhibitor of bone resorption by isolated osteoclasts. This Arg24-Gly25-Asp26-(RGD)-containing protein inhibited the excavation of bone slices by rat osteoclasts (IC50 = 0.1
L C Chuang et al.
Biochemical and biophysical research communications, 220(2), 246-254 (1996-03-18)
An echistatin analogue, designated as des(46-49)-[Ala8,37]-echistatin gamma, was synthesized chemically by solid-phase peptide synthesis. The analogue was made by replacing Cys8 and Cys37 residues with two alanines and the deletion of C-terminal peptide 46-49 of echistatin gamma, resulting in an
Functional and computational studies of the ligand-associated metal binding site of beta3 integrins.
Marta Murcia et al.
Proteins, 71(4), 1779-1791 (2008-01-05)
A combination of experimental and computational approaches was used to provide a structural context for the role of the beta3 integrin subunit ligand-associated metal binding site (LIMBS) in the binding of physiological ligands to beta3 integrins. Specifically, we have carried
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej