MTOX1020
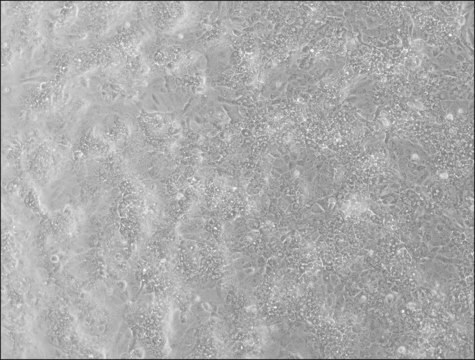
HepaRG™ Cells MRP2 (-/-)
human female liver (hepatocarcinoma and hepatitis C tumor)
About This Item
Polecane produkty
product name
HepaRG™ Cells MRP2 (-/-), one vial
pochodzenie biologiczne
human female liver (hepatocarcinoma and hepatitis C tumor)
Postać
liquid
numer dostępu OMIM
temp. przechowywania
−196°C
informacje o genach
human ... ABCC2(1244)
Opis ogólny
Zastosowanie
Cechy i korzyści
- The frame-shift mutation of ABCC2 gene was confirmed by fragment length analysis and DNA sequencing.
- Loss of functionality was confirmed by loss of transport of selective substrates in sandwich culture assay.
Jakość
Informacje prawne
Exhibit 2: HepaRG limited use license
Oświadczenie o zrzeczeniu się odpowiedzialności
Kod klasy składowania
12 - Non Combustible Liquids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Certyfikaty analizy (CoA)
Poszukaj Certyfikaty analizy (CoA), wpisując numer partii/serii produktów. Numery serii i partii można znaleźć na etykiecie produktu po słowach „seria” lub „partia”.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Produkty
Oral drug delivery involves dissolution in the small intestine and absorption across the enterocyte barrier into the portal vein followed by subsequent delivery through the liver into the systemic circulation.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej