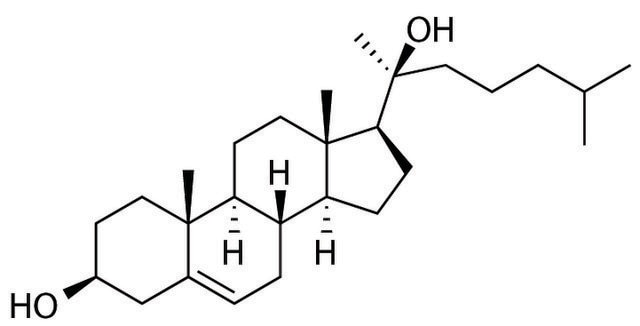
H6378
20α-Hydroxycholesterol
analytical standard
Synonim(y):
5-Cholestene-3β,20α-diol
About This Item
Polecane produkty
klasa czystości
analytical standard
Poziom jakości
Próba
≥98%
metody
HPLC: suitable
gas chromatography (GC): suitable
Zastosowanie
clinical testing
format
neat
ciąg SMILES
CC(C)CCC[C@](C)(O)[C@H]1CC[C@H]2[C@@H]3CC=C4C[C@@H](O)CC[C@]4(C)[C@H]3CC[C@]12C
InChI
1S/C27H46O2/c1-18(2)7-6-14-27(5,29)24-11-10-22-21-9-8-19-17-20(28)12-15-25(19,3)23(21)13-16-26(22,24)4/h8,18,20-24,28-29H,6-7,9-17H2,1-5H3/t20-,21-,22-,23-,24-,25-,26-,27-/m0/s1
Klucz InChI
MCKLJFJEQRYRQT-APGJSSKUSA-N
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Powiązane kategorie
Zastosowanie
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Środki ochrony indywidualnej
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Certyfikaty analizy (CoA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej