34218
Cefuroxime
VETRANAL®, analytical standard
About This Item
Polecane produkty
klasa czystości
analytical standard
Poziom jakości
linia produktu
VETRANAL®
okres trwałości
limited shelf life, expiry date on the label
metody
HPLC: suitable
gas chromatography (GC): suitable
Zastosowanie
clinical testing
format
neat
temp. przechowywania
2-8°C
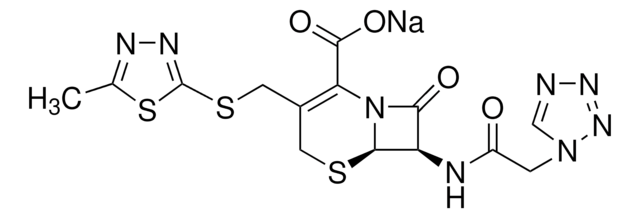
ciąg SMILES
[H][C@]12SCC(COC(N)=O)=C(N1C(=O)[C@H]2NC(=O)\C(=N/OC)c3ccco3)C(O)=O
InChI
1S/C16H16N4O8S/c1-26-19-9(8-3-2-4-27-8)12(21)18-10-13(22)20-11(15(23)24)7(5-28-16(17)25)6-29-14(10)20/h2-4,10,14H,5-6H2,1H3,(H2,17,25)(H,18,21)(H,23,24)/b19-9-/t10-,14-/m1/s1
Klucz InChI
JFPVXVDWJQMJEE-IZRZKJBUSA-N
Powiązane kategorie
Opis ogólny
Zastosowanie
Informacje prawne
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Środki ochrony indywidualnej
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej