557360
Roscovitine
≥95% (HPLC), solid, Cdk inhibitor, Calbiochem®
Synonim(y):
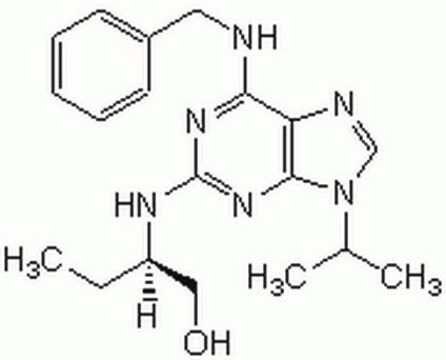
Roscovitine, 2-(R)-(1-Ethyl-2-hydroxyethylamino)-6-benzylamino-9-isopropylpurine, Cdk7 Inhibitor I
About This Item
Polecane produkty
product name
Roscovitine, A potent, reversible, and selective inhibitor of Cdks that exhibits about 10-fold greater efficacy towards p34-cdk1 and p33-cdk2 and 20-fold greater efficacy towards p33-cdk5 relative to Olomoucine.
Poziom jakości
Próba
≥95% (HPLC)
Postać
solid
producent / nazwa handlowa
Calbiochem®
warunki przechowywania
OK to freeze
kolor
white to off-white
rozpuszczalność
DMSO: 10 mg/mL
Warunki transportu
ambient
temp. przechowywania
−20°C
InChI
1S/C19H26N6O/c1-4-15(11-26)22-19-23-17(20-10-14-8-6-5-7-9-14)16-18(24-19)25(12-21-16)13(2)3/h5-9,12-13,15,26H,4,10-11H2,1-3H3,(H2,20,22,23,24)/t15-/m1/s1
Klucz InChI
BTIHMVBBUGXLCJ-OAHLLOKOSA-N
Opis ogólny
Działania biochem./fizjol.
p34cdk1/cyclin B
Ostrzeżenie
Rekonstytucja
Inne uwagi
Meijer, L., et al. 1997. Eur. J. Biochem.243, 527.
Meijer, L., et al. 1996. Trends Cell Biol. 6, 393.
Rudolph, B., et al. 1996. EMBO J. 15, 3053.
Informacje prawne
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Certyfikaty analizy (CoA)
Poszukaj Certyfikaty analizy (CoA), wpisując numer partii/serii produktów. Numery serii i partii można znaleźć na etykiecie produktu po słowach „seria” lub „partia”.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej