870706P
Avanti
06:0 Coenzyme A
Avanti Research™ - A Croda Brand 870706P, powder
Synonim(y):
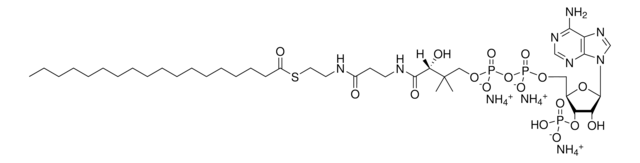
hexanoyl Coenzyme A (ammonium salt)
About This Item
Polecane produkty
Postać
powder
opakowanie
pkg of 1 × 5 mg (870706P-5mg)
producent / nazwa handlowa
Avanti Research™ - A Croda Brand 870706P
Zastosowanie
lipidomics
typ lipidu
coenzymes
Warunki transportu
dry ice
temp. przechowywania
−20°C
ciąg SMILES
O[C@@](C(NCCC(NCCSC(CCCCC)=O)=O)=O)(C(C)(COP([O-])(OP([O-])(OC[C@H]([C@H]1OP([O-])(O)=O)O[C@H]([C@@H]1O)N2C3=C(C(N)=NC=N3)N=C2)=O)=O)C)[H].[NH4+].[NH4+].[NH4+]
InChI
1S/C27H46N7O17P3S.3H3N/c1-4-5-6-7-18(36)55-11-10-29-17(35)8-9-30-25(39)22(38)27(2,3)13-48-54(45,46)51-53(43,44)47-12-16-21(50-52(40,41)42)20(37)26(49-16)34-15-33-19-23(28)31-14-32-24(19)34;;;/h14-16,20-22,26,37-38H,4-13H2,1-3H3,(H,29,35)(H,30,39)(H,43,44)(H,45,46)(H2,28,31,32)(H2,40,41,42);3*1H3/t16-,20?,21+,22+,26-;;;/m1.../s1
Klucz InChI
MXEFXWIYODKXEJ-WSHYHEJESA-N
Zastosowanie
Działania biochem./fizjol.
Opakowanie
Informacje prawne
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Certyfikaty analizy (CoA)
Poszukaj Certyfikaty analizy (CoA), wpisując numer partii/serii produktów. Numery serii i partii można znaleźć na etykiecie produktu po słowach „seria” lub „partia”.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej