700051P
Avanti
22(S)-hydroxycholesterol-d7
Avanti Research™ - A Croda Brand
Synonim(y):
25,26,26,26,27,27,27-heptadeuterocholest-5-ene-3β,22S-diol
Zaloguj sięWyświetlanie cen organizacyjnych i kontraktowych
About This Item
Wzór empiryczny (zapis Hilla):
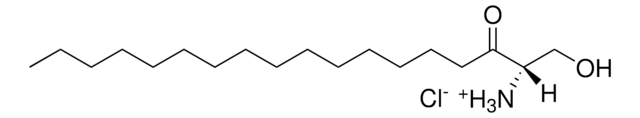
C27H39O2D7
Numer CAS:
Masa cząsteczkowa:
409.70
Kod UNSPSC:
41141804
NACRES:
NA.25
Polecane produkty
opis
cholest-5-ene-3β,22(S)-diol-d7
Próba
>99% (TLC)
Postać
powder
opakowanie
pkg of 1 × 1 mg (700051P-1mg)
producent / nazwa handlowa
Avanti Research™ - A Croda Brand
Warunki transportu
dry ice
temp. przechowywania
−20°C
Opis ogólny
22(S)-hydroxycholesterol is an enantiomer of 22(R)-hydroxycholesterol. 22(S)-hydroxycholesterol-d7 is a deuterated form of 22(S)-hydroxycholesterol.
Zastosowanie
22(S)-hydroxycholesterol-d7 may be used as an internal standard in liquid chromatography with tandem mass spectrometry (LC-MS-MS) analysis of plasma low-density lipoprotein (LDL).
Działania biochem./fizjol.
22(S)-hydroxycholesterol (22(S)-HC) promotes glucose catabolism and uptake and is regarded as a potential target to treat type 2 diabetes. 22(S)-HC also prevents the accumulation of lipids and lipid synthesis in hepatocytes and myotubes. Unlike 22(R)-hydroxycholesterol, 22(S)-HC is not estrogenic and is not a ligand for liver X receptor (LXR).
Opakowanie
5 mL Amber Glass Screw Cap Vial (700051P-1mg)
Informacje prawne
Avanti Research is a trademark of Avanti Polar Lipids, LLC
This page may contain text that has been machine translated.
Kod klasy składowania
11 - Combustible Solids
Certyfikaty analizy (CoA)
Poszukaj Certyfikaty analizy (CoA), wpisując numer partii/serii produktów. Numery serii i partii można znaleźć na etykiecie produktu po słowach „seria” lub „partia”.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Myung-Jin Oh et al.
Journal of lipid research, 57(5), 791-808 (2016-03-19)
Endothelial biomechanics is emerging as a key factor in endothelial function. Here, we address the mechanisms of endothelial stiffening induced by oxidized LDL (oxLDL) and investigate the role of oxLDL in lumen formation. We show that oxLDL-induced endothelial stiffening is
Hiroyoshi Sato et al.
Bioscience, biotechnology, and biochemistry, 68(8), 1790-1793 (2004-08-24)
In order to test the estrogenic activity of sterol oxidation products from cholesterol and phytosterols, an estrogen-dependent gene expression assay was performed in estrogen receptor alpha-stably transformed HeLa cells. The ranking of the estrogenic potency of these compounds was different:
Nina Pettersen Hessvik et al.
The Journal of steroid biochemistry and molecular biology, 128(3-5), 154-164 (2011-11-05)
The aim of this study was to explore the effects of 22(S)-hydroxycholesterol (22(S)-HC) on lipid and glucose metabolism in human-derived cells from metabolic active tissues. Docking of T0901317 and 22(S)-HC showed that both substances fitted into the ligand binding domain
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej