907294
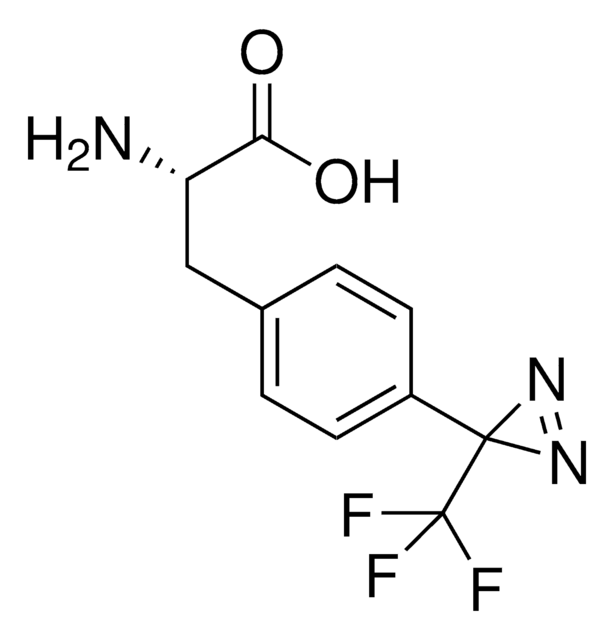
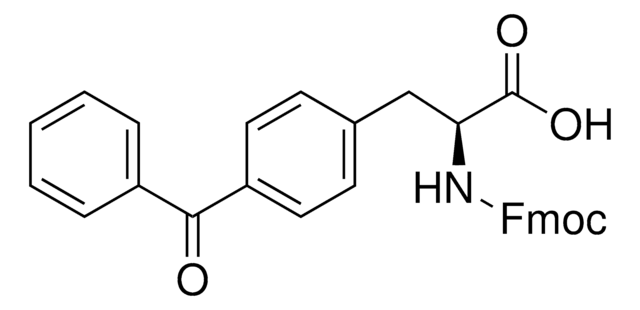
Fmoc-L-Photo-Phe-OH
≥95%
别名:
(S)-2-((((9H-Fluoren-9-yl)methoxy)carbonyl)amino)-3-(4-(3-(trifluoromethyl)-3H-diazirin-3-yl)phenyl)propanoic acid, N-α-(9-Fluorenylmethyloxycarbonyl)-4-(trifluoromethyldiazirin)-L-phenylalanine, Diazirine amino acid, Fmoc-Tdf-OH, Photo-Phe, Photo-crosslinking amino acid, Photoprobe building block
登录查看公司和协议定价
所有图片(2)
About This Item
推荐产品
化驗
≥95%
形狀
powder
反應適用性
reaction type: Fmoc solid-phase peptide synthesis
應用
peptide synthesis
官能基
Fmoc
儲存溫度
−20°C
應用
Fmoc-L-Photo-Phe-OH is a diazirine-containing, Fmoc-protected phenylalanine amino acid and multifunctional photo-crosslinker. Its incorporation into peptides or small-molecule probes and tools allows for photoaffinity labeling of cellular targets and protein-protein interactions upon UV light (∼360 nm) irradiation to form a covalent bond. This and other multifunctional probe building blocks will continue to accelerate drug discovery research for probing cellular mechanisms, target ID/validation, and understanding traditionally undruggable targets. An unprotected version is also available as 907340.
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
其他說明
Covalent modifier-type aggregation inhibitor of amyloid-β based on a cyclo-KLVFF motif
Mode of Action of cGMP-dependent Protein Kinase-specific Inhibitors Probed by Photoaffinity Cross-linking Mass Spectrometry
Trifluoromethyldiazirine: an effective photo-induced cross-linking probe for exploring amyloid formation
Simple and Versatile Method for Tagging Phenyldiazirine Photophores
Fishing for Drug Targets: A Focus on Diazirine Photoaffinity Probe Synthesis
Photo-affinity labeling (PAL) in chemical proteomics: a handy tool to investigate protein-protein interactions (PPIs)
Mode of Action of cGMP-dependent Protein Kinase-specific Inhibitors Probed by Photoaffinity Cross-linking Mass Spectrometry
Trifluoromethyldiazirine: an effective photo-induced cross-linking probe for exploring amyloid formation
Simple and Versatile Method for Tagging Phenyldiazirine Photophores
Fishing for Drug Targets: A Focus on Diazirine Photoaffinity Probe Synthesis
Photo-affinity labeling (PAL) in chemical proteomics: a handy tool to investigate protein-protein interactions (PPIs)
相關產品
产品编号
说明
价格
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
Michiel A Leeuwenburgh et al.
Organic letters, 8(8), 1705-1708 (2006-04-07)
[reaction: see text] A novel solid-phase synthesis strategy toward succinylhydroxamate peptides, using an appropriately protected hydroxamate building block, is described. Rapid and efficient access is gained to amine-functionalized peptides, which can be decorated with, for instance, a fluorescent label. In
Ryuto Kino et al.
Bioorganic & medicinal chemistry letters, 25(15), 2972-2975 (2015-06-06)
Inhibition of amyloid-β (Aβ) aggregation could be a drug development target for treating Alzheimer disease. Insufficient activity to inhibit aggregation, however, remains a key issue. Here, we report a covalent modifier-type aggregation inhibitor of Aβ, diazirine-equipped cyclo-KLVF(β-Ph)F (2). Due to
Dany Fillion et al.
Journal of medicinal chemistry, 49(7), 2200-2209 (2006-03-31)
A stereospecific convergent synthesis of N-[(9-fluorenyl)methoxycarbonyl]-p-[3-(trifluoromethyl)-3H-diazirin-3-yl]-l-phenylalanine (Fmoc-12, Fmoc-Tdf) and its incorporation into the C-terminal position of the angiotensin II (AngII) peptide to form (125)I[Sar(1),Tdf(8)]AngII ((125)I-13) is presented. This amino acid photoprobe is a highly reactive carbene-generating diazirine phenylalanine derivative that
M Ploug et al.
Biochemistry, 37(11), 3612-3622 (1998-04-02)
Binding of urokinase-type plasminogen activator (uPA) to its cellular receptor (uPAR) renders the cell surface a favored site for plasminogen activation. Recently, a 15-mer peptide antagonist of the uPA-uPAR interaction, with an IC50 value of 10 nM, was identified using
R Falchetto et al.
The Journal of biological chemistry, 266(5), 2930-2936 (1991-02-15)
A synthetic, 28-residue peptide derived from the calmodulin-binding sequence of the plasma membrane Ca2+ pump (C28W) inhibits the ATPase activity of a calpain-produced, truncated fragment of the enzyme. The fragment, which has lost the calmodulin-binding domain, has a molecular mass
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门