推荐产品
化驗
≥98%
形狀
powder
反應適用性
reaction type: Fmoc solid-phase peptide synthesis
應用
peptide synthesis
官能基
Fmoc
儲存溫度
2-8°C
應用
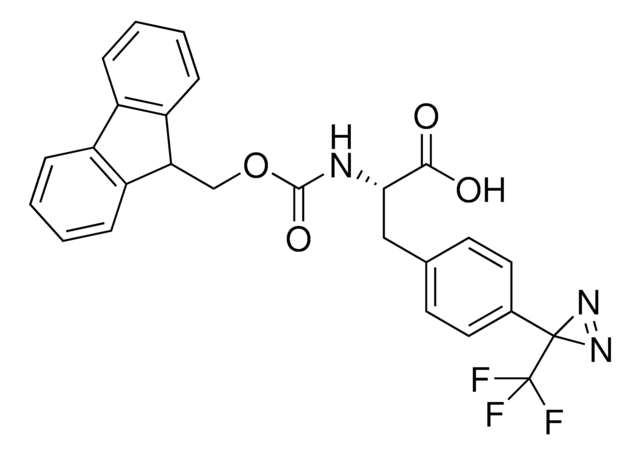
Fmoc-L-Photo-Leucine is a diazirine-containing, Fmoc-protected leucine amino acid and multifunctional photo-crosslinker. Its incorporation into peptides or small-molecule probes and tools allows for photoaffinity labeling of cellular targets and protein-protein interactions upon UV light (∼360 nm) irradiation to form a covalent bond. This and other multifunctional probe building blocks will continue to accelerate drug discovery research for probing cellular mechanisms, target ID/validation, and understanding traditionally undruggable targets. An unprotected version is also available as 907278.
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
其他說明
Synthesis of a polymyxin derivative for photolabeling studies in the gram-negative bacterium Escherichia coli
Developing diazirine-based chemical probes to identify histone modification ′readers′ and ′erasers′
Protein-Polymer Conjugation via Ligand Affinity and Photoactivation of Glutathione S-Transferase
Fishing for Drug Targets: A Focus on Diazirine Photoaffinity Probe Synthesis
Photo-affinity labeling (PAL) in chemical proteomics: a handy tool to investigate protein-protein interactions (PPIs)
Developing diazirine-based chemical probes to identify histone modification ′readers′ and ′erasers′
Protein-Polymer Conjugation via Ligand Affinity and Photoactivation of Glutathione S-Transferase
Fishing for Drug Targets: A Focus on Diazirine Photoaffinity Probe Synthesis
Photo-affinity labeling (PAL) in chemical proteomics: a handy tool to investigate protein-protein interactions (PPIs)
相關產品
产品编号
说明
价格
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Aleš Marek et al.
Journal of the American Society for Mass Spectrometry, 25(5), 778-789 (2014-02-20)
Gas-phase dissociations were investigated for several peptide ions containing the Gly-Leu* N-terminal motif where Leu* was a modified norleucine residue containing the photolabile diazirine ring. Collisional activation of gas-phase peptide cations resulted in facile N₂ elimination that competed with backbone
Benjamin van der Meijden et al.
Journal of peptide science : an official publication of the European Peptide Society, 21(3), 231-235 (2015-02-03)
The antimicrobial activity of polymyxins against Gram-negative bacteria has been known for several decades, but the mechanism of action leading to cell death has not been fully explored. A key step after binding of the antibiotic to lipopolysaccharide (LPS) exposed
En-Wei Lin et al.
Bioconjugate chemistry, 25(10), 1902-1909 (2014-10-16)
A photoactivated, site-selective conjugation of poly(ethylene glycol) (PEG) to the glutathione (GSH) binding pocket of glutathione S-transferase (GST) is described. To achieve this, a GSH analogue (GSH-BP) was designed and chemically synthesized with three functionalities: (1) the binding affinity of
Developing diazirine-based chemical probes to identify histone modification 'readers' and 'erasers'.
Tangpo Yang et al.
Chemical science, 6(2), 1011-1017 (2015-02-01)
Post translational modifications (PTMs, e.g., phosphorylation, acetylation and methylation) of histone play important roles in regulating many fundamental cellular processes such as gene transcription, DNA replication and damage repair. While 'writer' and 'eraser' enzymes modify histones by catalyzing the addition
Jay M Janz et al.
Journal of the American Chemical Society, 133(40), 15878-15881 (2011-09-13)
Cell surface heptahelical G protein-coupled receptors (GPCRs) mediate critical cellular signaling pathways and are important pharmaceutical drug targets. (1) In addition to traditional small-molecule approaches, lipopeptide-based GPCR-derived pepducins have emerged as a new class of pharmaceutical agents. (2, 3)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门