推荐产品
化驗
≥98%
形狀
powder
反應適用性
reaction type: solution phase peptide synthesis
存貨情形
available only in USA
應用
peptide synthesis
儲存溫度
−20°C
InChI
1S/C11H10F3N3O2/c12-11(13,14)10(16-17-10)7-3-1-6(2-4-7)5-8(15)9(18)19/h1-4,8H,5,15H2,(H,18,19)
InChI 密鑰
HRGXDARRSCSGOG-UHFFFAOYSA-N
應用
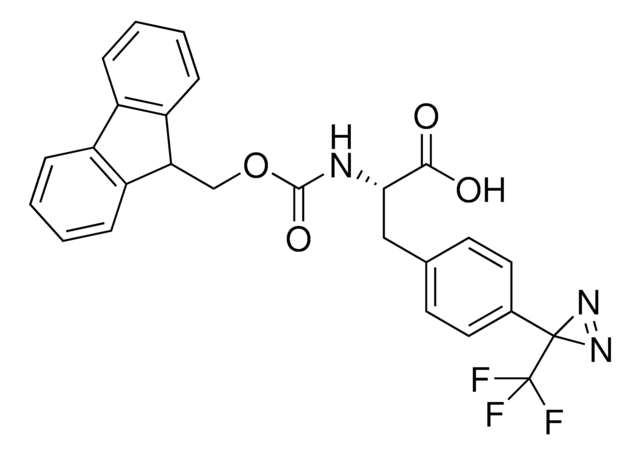
H-L-Photo-Phe-OH is a diazirine-containing phenylalanine amino acid and multifunctional
photo-crosslinker. Its incorporation into peptides or small-molecule probes and tools allows for photoaffinity labeling of cellular targets and protein-protein interactions upon UV light (∼360 nm) irradiation to form a covalent bond. This and other multifunctional probe building blocks will continue to accelerate drug discovery research for probing cellular mechanisms, target ID/validation, and understanding traditionally undruggable targets. An Fmoc-protected version is also available as 907294.
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
photo-crosslinker. Its incorporation into peptides or small-molecule probes and tools allows for photoaffinity labeling of cellular targets and protein-protein interactions upon UV light (∼360 nm) irradiation to form a covalent bond. This and other multifunctional probe building blocks will continue to accelerate drug discovery research for probing cellular mechanisms, target ID/validation, and understanding traditionally undruggable targets. An Fmoc-protected version is also available as 907294.
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
其他說明
Genetic Incorporation of a Photo-Crosslinkable Amino Acid Reveals Novel Protein Complexes with GRB2 in Mammalian Cells
Trifluoromethyldiazirine: an effective photo-induced cross-linking probe for exploring amyloid formation
A genetically encoded diazirine photo-crosslinker in Escherichia coli
Fishing for Drug Targets: A Focus on Diazirine Photoaffinity Probe Synthesis
Photo-affinity labeling (PAL) in chemical proteomics: a handy tool to investigate protein-protein interactions (PPIs)
Trifluoromethyldiazirine: an effective photo-induced cross-linking probe for exploring amyloid formation
A genetically encoded diazirine photo-crosslinker in Escherichia coli
Fishing for Drug Targets: A Focus on Diazirine Photoaffinity Probe Synthesis
Photo-affinity labeling (PAL) in chemical proteomics: a handy tool to investigate protein-protein interactions (PPIs)
相關產品
产品编号
说明
价格
訊號詞
Danger
危險聲明
危險分類
Self-react. C
儲存類別代碼
5.2 - Organic peroxides and self-reacting hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
A genetically encoded diazirine photocrosslinker in Escherichia coli.
Eric M Tippmann et al.
Chembiochem : a European journal of chemical biology, 8(18), 2210-2214 (2007-11-15)
G Baldini et al.
Biochemistry, 27(20), 7951-7959 (1988-10-04)
The Boc-protected derivative of a photoactivatable, carbene-generating analogue of phenylalanine, L-4'-[3-(trifluoromethyl)-3H-diazirin-3-yl]phenylalanine [(Tmd)Phe], was used to acylate 5'-O-phosphorylcytidylyl(3'-5')adenosine (pCpA). A diacyl species was isolated which upon successive treatments with trifluoroacetic acid and 0.01 M HCl yielded a 1:1 mixture of 2'(3')-O-(Tmd)phenylalanyl-pCpA
Nobumasa Hino et al.
Journal of molecular biology, 406(2), 343-353 (2010-12-28)
Cell signaling pathways are essentially organized through the distribution of various types of binding domains in signaling proteins, with each domain binding to specific target molecules. Although identification of these targets is crucial for mapping the pathways, affinity-based or copurification
Lei Wang et al.
Molecules (Basel, Switzerland), 18(7), 8393-8401 (2013-07-19)
Photoaffinity labeling is a reliable analytical method for biological functional analysis. Three major photophores--aryl azide, benzophenone and trifluoromethyldiazirine--are utilized in analysis. Photophore-bearing L-phenylalanine derivatives, which are used for biological functional analysis, were inoculated into a Klebsiella sp. isolated from the
Lei Wang et al.
Bioscience, biotechnology, and biochemistry, 78(7), 1129-1134 (2014-09-18)
In this paper we report here a hydrogen/deuterium exchange (H/D exchange) of cross-linkable α-amino acid derivatives with deuterated trifluoromethanesulfonic acid (TfOD). H/D exchange with TfOD was easily applied to o-catechol containing phenylalanine (DOPA) within an hour. A partial H/D exchange
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门