T2057
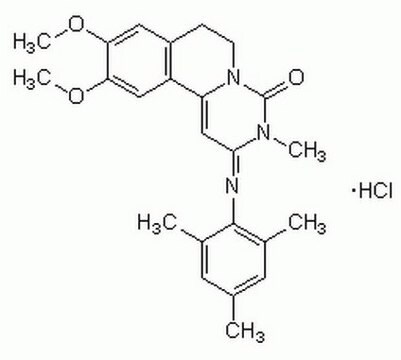
Trequinsin hydrochloride
≥98%
Sinonimo/i:
HL 725
Scegli un formato
270,90 €
Prezzo di listino387,00 €Risparmia il 30%Scegli un formato
About This Item
270,90 €
Prezzo di listino387,00 €Risparmia il 30%Prodotti consigliati
Saggio
≥98%
Stato
powder
Stringa SMILE
Cl[H].COc1cc2CCN3C(=O)N(C)C(\C=C3c2cc1OC)=N\c4c(C)cc(C)cc4C
InChI
1S/C24H27N3O3.ClH/c1-14-9-15(2)23(16(3)10-14)25-22-13-19-18-12-21(30-6)20(29-5)11-17(18)7-8-27(19)24(28)26(22)4;/h9-13H,7-8H2,1-6H3;1H/b25-22+;
DTCZZBVPTHVXFA-OSMRDGEFSA-N
Informazioni sul gene
human ... PDE3A(5139) , PDE3B(5140)
Applicazioni
Azioni biochim/fisiol
Caratteristiche e vantaggi
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Articoli
Cyclic nucleotides like cAMP modulate cell function via PKA activation and ion channels.
Filtri attivi
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.