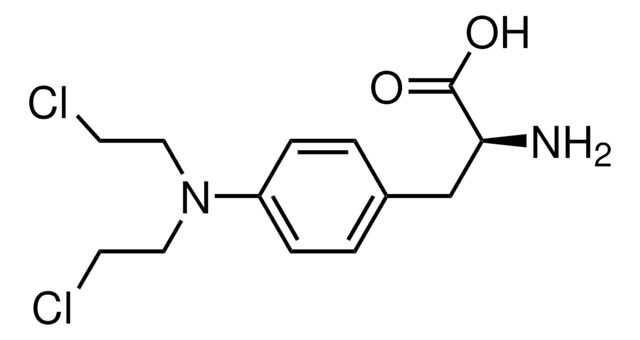
A4330
4′-Aminomethyltrioxsalen hydrochloride
Sinonimo/i:
4′-Aminomethyl-4,5′,8-trimethylpsoralen hydrochloride
About This Item
Prodotti consigliati
Forma fisica
powder
Livello qualitativo
Solubilità
H2O: 1 mg/mL
DMSO: 2 mg/mL
Temperatura di conservazione
2-8°C
Stringa SMILE
CC1=CC(=O)Oc2c(C)c3oc(C)c(CN)c3cc12
InChI
1S/C15H15NO3/c1-7-4-13(17)19-14-8(2)15-11(5-10(7)14)12(6-16)9(3)18-15/h4-5H,6,16H2,1-3H3
WBIICVGYYRRURR-UHFFFAOYSA-N
Applicazioni
Azioni biochim/fisiol
Caratteristiche e vantaggi
Avvertenze
Danger
Indicazioni di pericolo
Classi di pericolo
Acute Tox. 4 Oral - Carc. 2 - Skin Corr. 1B
Codice della classe di stoccaggio
8A - Combustible corrosive hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
DNA damage and repair mechanism is vital for maintaining DNA integrity. Damage to cellular DNA is involved in mutagenesis, the development of cancer among others.
Cell cycle phases (G1, S, G2, M) regulate cell growth, DNA replication, and division in proliferating cells.
Apoptosis regulation involves multiple pathways and molecules for cellular homeostasis.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.