Scegli un formato
Informazioni su questo articolo
Vai a
form
powder
Quality Level
SMILES string
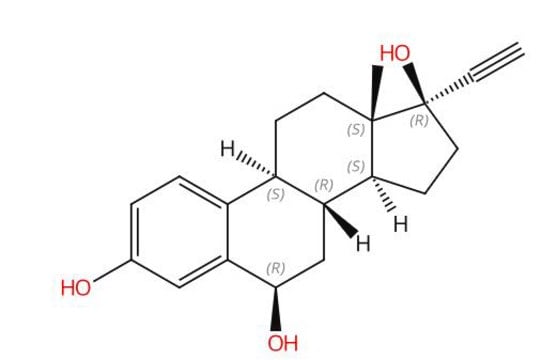
O[C@@H]1C([C@H]2[C@@]3([C@]4([C@H]([C@]5([C@@]([C@H](CC5)[C@@H](CCC=C(C)C)C)(CC4)C)C)CC2)C3)CC1)(C)C
InChI
1S/C30H50O/c1-20(2)9-8-10-21(3)22-13-15-28(7)24-12-11-23-26(4,5)25(31)14-16-29(23)19-30(24,29)18-17-27(22,28)6/h9,21-25,31H,8,10-19H2,1-7H3/t21-,22-,23+,24+,25+,27-,28+,29-,30+/m1/s1
InChI key
ONQRKEUAIJMULO-YBXTVTTCSA-N
assay
≥90% (GC)
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
1 of 4
Questo articolo | |||
|---|---|---|---|
| assay ≥90% (GC) | assay ≥90.0% (HPLC) | assay - | assay - |
| form powder | form powder | form - | form - |
| Quality Level 100 | Quality Level - | Quality Level - | Quality Level - |
Application
- Genome-Wide Investigation of Oxidosqualene Cyclase Genes Deciphers the Genetic Basis of Triterpene Biosynthesis in Tea Plants - Research on cycloartenol′s synthesis pathways through the genetic study of oxidosqualene cyclase in tea plants, providing insights into the enhancement of plant sterols beneficial for human health (Du et al., 2024).
Packaging
Classe di stoccaggio
11 - Combustible Solids
wgk
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Filtri attivi
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica