Fontos dokumentumok
HPA006801
Anti-XRCC4 antibody produced in rabbit

Prestige Antibodies® Powered by Atlas Antibodies, affinity isolated antibody, buffered aqueous glycerol solution
Szinonimák:
Anti-DNA-repair protein XRCC4, Anti-X-ray repair cross-complementing protein 4
About This Item
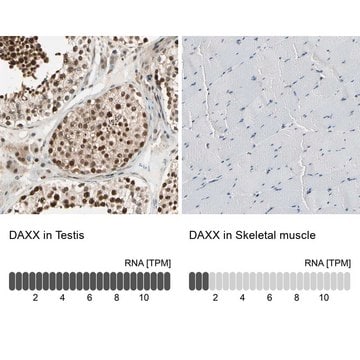
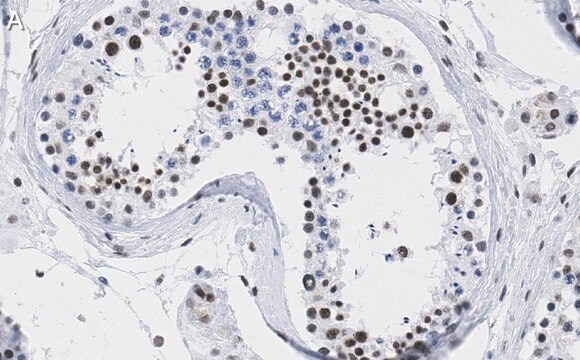
IHC
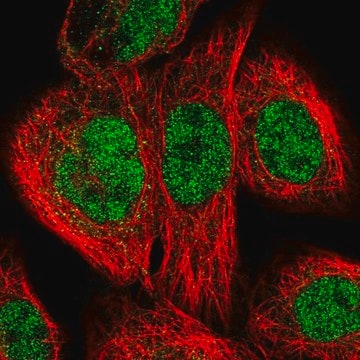
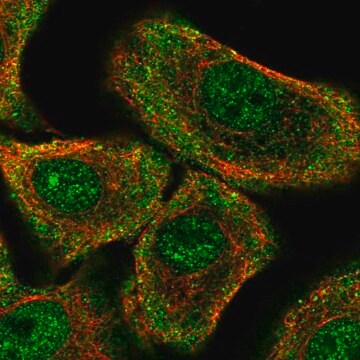
immunofluorescence: 0.25-2 μg/mL
immunohistochemistry: 1:500-1:1000
Javasolt termékek
biológiai forrás
rabbit
Minőségi szint
konjugátum
unconjugated
antitest forma
affinity isolated antibody
antitest terméktípus
primary antibodies
klón
polyclonal
termékcsalád
Prestige Antibodies® Powered by Atlas Antibodies
Forma
buffered aqueous glycerol solution
faj reaktivitás
human
fejlettebb validálás
RNAi knockdown
orthogonal RNAseq
recombinant expression
Learn more about Antibody Enhanced Validation
technika/technikák
immunoblotting: 0.04-0.4 μg/mL
immunofluorescence: 0.25-2 μg/mL
immunohistochemistry: 1:500-1:1000
immunogén szekvencia
KCVSAKEALETDLYKRFILVLNEKKTKIRSLHNKLLNAAQEREKDIKQEGETAICSEMTADRDPVYDESTDEESENQTDLSGLASAAVSKDDSIISSLDVTDIAPSRKRRQRMQRNLGTEPKMAPQENQLQEK
UniProt elérési szám
kiszállítva
wet ice
tárolási hőmérséklet
−20°C
célzott transzláció utáni módosítás
unmodified
Géninformáció
human ... XRCC4(7518)
Immunogén
Alkalmazás
Western Blotting (1 paper)
Biokémiai/fiziológiai hatások
Tulajdonságok és előnyök
Every Prestige Antibody is tested in the following ways:
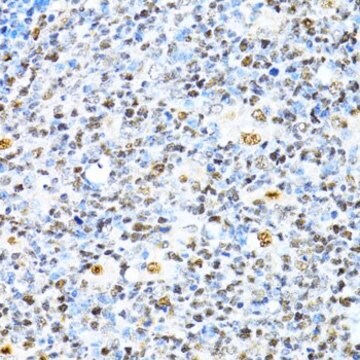
- IHC tissue array of 44 normal human tissues and 20 of the most common cancer type tissues.
- Protein array of 364 human recombinant protein fragments.
Kapcsolódás
Fizikai forma
Jogi információk
Jogi nyilatkozat
Nem találja a megfelelő terméket?
Próbálja ki a Termékválasztó eszköz. eszközt
Tárolási osztály kódja
10 - Combustible liquids
WGK
WGK 1
Lobbanási pont (F)
Not applicable
Lobbanási pont (C)
Not applicable
Egyéni védőeszköz
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
Válasszon a legfrissebb verziók közül:
Analitikai tanúsítványok (COA)
Nem találja a megfelelő verziót?
Ha egy adott verzióra van szüksége, a tétel- vagy cikkszám alapján rákereshet egy adott tanúsítványra.
Már rendelkezik ezzel a termékkel?
Az Ön által nemrégiben megvásárolt termékekre vonatkozó dokumentumokat a Dokumentumtárban találja.
Tudóscsoportunk valamennyi kutatási területen rendelkezik tapasztalattal, beleértve az élettudományt, az anyagtudományt, a kémiai szintézist, a kromatográfiát, az analitikát és még sok más területet.
Lépjen kapcsolatba a szaktanácsadással