MABN2287
Anti-APP Antibody, clone 1D1
clone 1D1, from rat
Szinonimák:
Amyloid beta A4 protein, ABPP, APPI, Alzheimer disease amyloid protein, Amyloid precursor protein, Beta-amyloid precursor protein, Cerebral vascular amyloid peptide, CVAP, PreA4, Protease nexin-II, PN-II
About This Item
FACS
ICC
IHC
WB
flow cytometry: suitable
immunocytochemistry: suitable
immunohistochemistry: suitable (paraffin)
western blot: suitable
Javasolt termékek
biológiai forrás
rat
Minőségi szint
antitest forma
purified immunoglobulin
antitest terméktípus
primary antibodies
klón
1D1, monoclonal
faj reaktivitás
human
technika/technikák
ELISA: suitable
flow cytometry: suitable
immunocytochemistry: suitable
immunohistochemistry: suitable (paraffin)
western blot: suitable
izotípus
IgG1κ
NCBI elérési szám
UniProt elérési szám
kiszállítva
ambient
célzott transzláció utáni módosítás
unmodified
Géninformáció
human ... APP(351)
Általános leírás
Egyediség
Immunogén
Alkalmazás
Immunoprecipitation Analysis: A representative lot detected APP in Immunoprecipitation applications (Hofling, C., et. al. (2016). Aging Cell. 15(5):953-63).
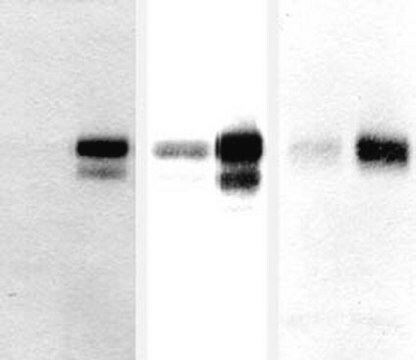
Western Blotting Analysis: A representative lot detected APP in WT, but not in APP knockdown HEK293T cells (Courtesy of Dr. med. Peer-Hendrik Kuhn, Ph.D., Institut fur Allgemeine Pathologie und Pathologische Anatomie, Technische Universität München, Munich, Germany).
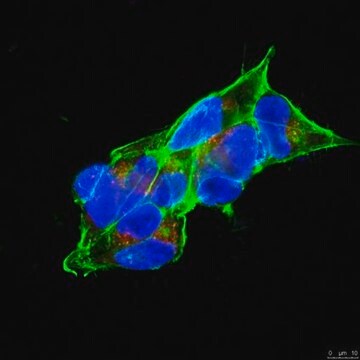
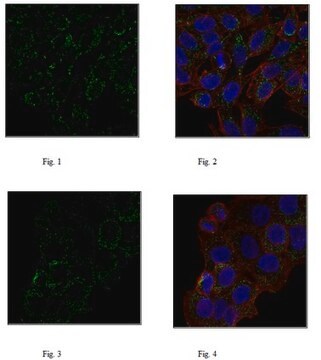
Immunocytochemistry Analysis: A 1:250 dilution from a representative lot detected APP in HEK293 cell line.
Immunocytochemistry Analysis: A representative lot detected APP in Immunocytochemistry applications (Hofling, C., et. al. (2016). Aging Cell. 15(5):953-63).
Immunocytochemistry Analysis: A 1:10 dilution from a representative lot detected APP in HEK293T cells, but not in cells lentivirally transduced with APP shRNA-1 or APP shRNA-2, both coexpressing GFP as a reporter (Courtesy of Dr. med. Peer-Hendrik Kuhn, Ph.D., Institut fur Allgemeine Pathologie und Pathologische Anatomie, Technische Universität München, Munich, Germany).
Immunohistochemistry Analysis: A representative lot detected APP in Immunohistochemistry applications (Hofling, C., et. al. (2016). Aging Cell. 15(5):953-63).
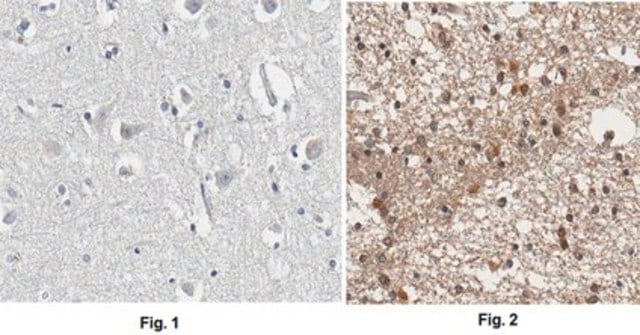
Immunohistochemistry Analysis: A 1:50 dilution from a representative lot detected APP in human cerebral cortex and human Alzheimer′s brain tissues.
ELISA Analysis: A representative lot detected APP in ELISA applications (Hofling, C., et. al. (2016). Aging Cell. 15(5):953-63).
Western Blotting Analysis: A representative lot detected APP in Western Blotting applications (Hofling, C., et. al. (2016). Aging Cell. 15(5):953-63).
Flow Cytometry Analysis: A representative lot detected APP in Flow Cytometry applications (Hofling, C., et. al. (2016). Aging Cell. 15(5):953-63).
Neuroscience
Minőség
Western Blotting Analysis: 1 µg/mL of this antibody detected APP in 10 µg of HEK293 cell lysate.
Cél megnevezése
Fizikai forma
Tárolás és stabilitás
Egyéb megjegyzések
Jogi nyilatkozat
Nem találja a megfelelő terméket?
Próbálja ki a Termékválasztó eszköz. eszközt
javasolt
Tárolási osztály kódja
12 - Non Combustible Liquids
WGK
WGK 1
Lobbanási pont (F)
does not flash
Lobbanási pont (C)
does not flash
Analitikai tanúsítványok (COA)
Analitikai tanúsítványok (COA) keresése a termék sarzs-/tételszámának megadásával. A sarzs- és tételszámok a termék címkéjén találhatók, a „Lot” vagy „Batch” szavak után.
Már rendelkezik ezzel a termékkel?
Az Ön által nemrégiben megvásárolt termékekre vonatkozó dokumentumokat a Dokumentumtárban találja.
Tudóscsoportunk valamennyi kutatási területen rendelkezik tapasztalattal, beleértve az élettudományt, az anyagtudományt, a kémiai szintézist, a kromatográfiát, az analitikát és még sok más területet.
Lépjen kapcsolatba a szaktanácsadással