07-018-I
Anti-phospho-p70 S6 Kinase (Thr389) Antibody
from rabbit, purified by affinity chromatography
Szinonimák:
Ribosomal protein S6 kinase, S6K-beta-1, S6K1, p70S6K1, p70 S6K1, p70-S6K1, p70 S6KA
About This Item
inhibition assay
western blot: suitable
Javasolt termékek
biológiai forrás
rabbit
Minőségi szint
antitest forma
affinity isolated antibody
antitest terméktípus
primary antibodies
klón
polyclonal
tisztítva
affinity chromatography
faj reaktivitás
human
faj reaktivitás (homológia által előrejelzett)
zebrafish (based on 100% sequence homology), chicken (based on 100% sequence homology), rat (based on 100% sequence homology), bovine (based on 100% sequence homology), mouse (based on 100% sequence homology)
technika/technikák
inhibition assay: suitable (peptide)
western blot: suitable
NCBI elérési szám
UniProt elérési szám
kiszállítva
wet ice
célzott transzláció utáni módosítás
phosphorylation (pThr389)
Géninformáció
human ... RPS6KB1(6198)
Általános leírás
Egyediség
Immunogén
phosphorylated at Thr389.
Alkalmazás
Signaling
Kinases & Phosphatases
Minőség
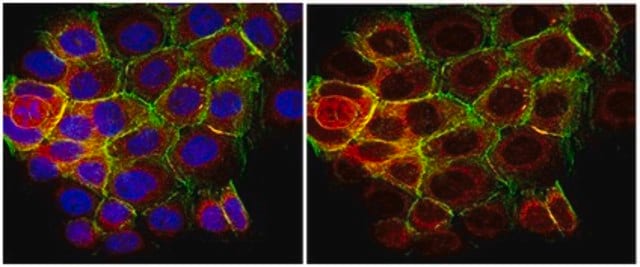
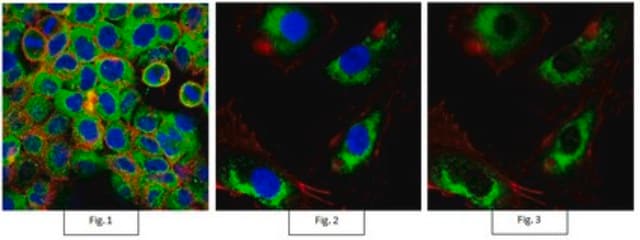
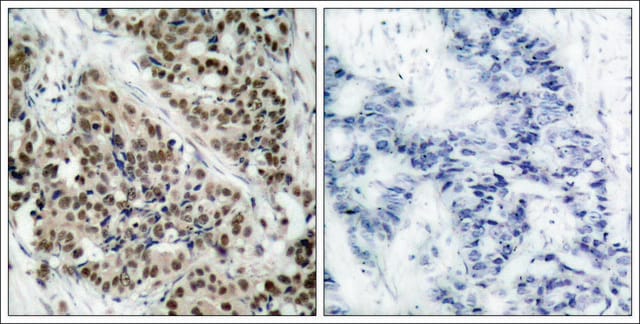
Western Blot (Peptide Inhibition) Analysis: 1.0 µg/mL of this antibody detected p70 S6 Kinase phosphorylated at Thr389 in 10 µg of MCF7 cell lysate and MCF7 cell lysate incubated with a non-phospho peptide.
Cél megnevezése
Fizikai forma
Tárolás és stabilitás
Analízis megjegyzés
MCF7 cell lysate
Egyéb megjegyzések
Jogi nyilatkozat
Nem találja a megfelelő terméket?
Próbálja ki a Termékválasztó eszköz. eszközt
Tárolási osztály kódja
12 - Non Combustible Liquids
WGK
WGK 1
Lobbanási pont (F)
Not applicable
Lobbanási pont (C)
Not applicable
Analitikai tanúsítványok (COA)
Analitikai tanúsítványok (COA) keresése a termék sarzs-/tételszámának megadásával. A sarzs- és tételszámok a termék címkéjén találhatók, a „Lot” vagy „Batch” szavak után.
Már rendelkezik ezzel a termékkel?
Az Ön által nemrégiben megvásárolt termékekre vonatkozó dokumentumokat a Dokumentumtárban találja.
Tudóscsoportunk valamennyi kutatási területen rendelkezik tapasztalattal, beleértve az élettudományt, az anyagtudományt, a kémiai szintézist, a kromatográfiát, az analitikát és még sok más területet.
Lépjen kapcsolatba a szaktanácsadással