推荐产品
化驗
≥99%
形狀
powder
mp
>300 °C (dec.) (lit.)
溶解度
DMF: soluble 5%, clear, colorless to yellow
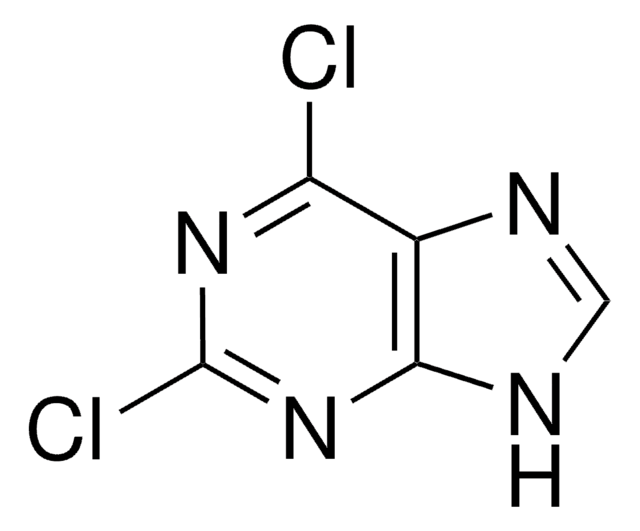
SMILES 字串
Clc1ncnc2[nH]cnc12
InChI
1S/C5H3ClN4/c6-4-3-5(9-1-7-3)10-2-8-4/h1-2H,(H,7,8,9,10)
InChI 密鑰
ZKBQDFAWXLTYKS-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
已研究 6-氯嘌呤与 3,4-二--O -乙酰基--D -木醛的酸催化反应。
應用
6-氯嘌呤在 DMSO 中通过各种取代卤代烷烃的烷基化反应制备 9-烷基嘌呤。也用于 6-琥珀氨基嘌呤的制备。
其他客户在看
Synthesis of Potential Anticancer Agents. XXVI. The Alkylation of 6-Chloropurine2.
Montgomery JA and Temple Jr C.
Journal of the American Chemical Society, 83(3), 630-635 (1961)
Synthesis of 6-succinoaminopurine.
C E CARTER
The Journal of biological chemistry, 223(1), 139-146 (1956-11-01)
Heterocyclic N-glycosides-V: Synthesis of unsaturated N-glycosides from 6-chloropurine and derivatives of d-xylal and l-arabinal. A conformational NMR study.
Fuertes M, et al.
Tetrahedron, 26(20), 4823-4837 (1970)
V Gurvich et al.
Nucleosides & nucleotides, 18(10), 2327-2333 (2000-01-05)
Tetrabutylammonium triphenydifluorosilicate (TBAT) has been found to be a useful reagent for the conversion of 6-chloropurine nucleosides to 6-fluoropurine derivatives. The 6-chloropurine nucleosides were reacted with trimethylamine to form quaternary trimethylammonium salts which were treated in situ with TBAT in
Dong-Chao Wang et al.
Organic & biomolecular chemistry, 9(22), 7663-7666 (2011-09-22)
An efficient method for the synthesis of 6-alkyl or 6-aryl purines (nucleosides) was developed via nickel-catalyzed Negishi cross-couplings of 6-chloropurines and organozinc halides. The ligand-free process gave good to excellent isolated yields at room temperature.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门