P2645
Protein Kinase A Catalytic Subunit from bovine heart
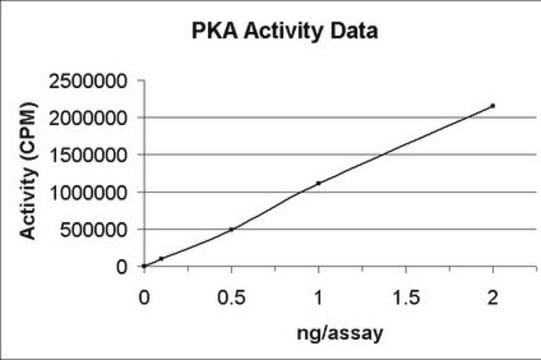
≥9 units/μg protein (cyclic-AMP is not required for this activity), lyophilized (white powder to sticky mass to hard pellet)
Synonyma:
PKA
Přihlásitk zobrazení cen stanovených pro organizaci a smluvních cen
About This Item
MDL number:
UNSPSC Code:
12352204
eCl@ss:
32160410
NACRES:
NA.32
Doporučené produkty
form
lyophilized (white powder to sticky mass to hard pellet)
Quality Level
specific activity
≥9 units/μg protein (cyclic-AMP is not required for this activity)
mol wt
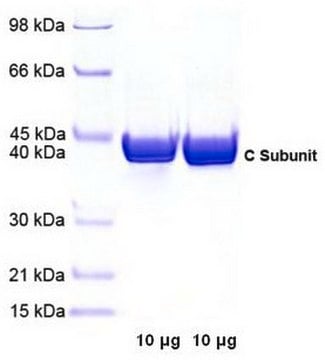
40,862 Da
storage temp.
−20°C
General description
Protein Kinase A enzyme is composed of two subunits- catalytic and regulatory. The catalytic subunit exists as a monomer in the presence of cAMP and has a molecular weight of 40,862 Da.
Application
Protein Kinase A (PKA) Catalytic Subunit from bovine heart has been used-
- to study PKA-mediated inhibition of IRK1 (inwardly rectifying K+) channels
- in in Vitro PKA pPhosphorylation assay
- in Vitro affinity binding assays
- to study effects of PKA on inspiratory drive currents in functionally active motorneurons
Biochem/physiol Actions
Protein Kinase A (PKA) controls the transduction of Hedgehog signaling and participates in proliferation and fate specification. It phosphorylates several neurotransmitter receptors, transcription factors and constituents of various intracellular signaling pathways.
Protein Kinase A catalyzes the transfer of terminal phosphate from ATP to threonine or serine residues present on various proteins. This protein is inactive in the absence of cAMP, where the catalytic and regulatory subunits are bound together. The regulatory subunit, in the presence of cAMP, binds to cAMP and releases the catalytic subunit.
Packaging
Package size based on phosphorylating units
Unit Definition
Phosphorylating Activity: One unit will transfer 1.0 picomole phosphate from ATP to hydrolyzed and partially dephosphorylated casein per minute at pH 6.5 at 30°C, determined by measuring the production of ADP.
Physical form
Lyophilized powder with sucrose and phosphate buffer salts as stabilizer.
Preparation Note
Prepared from protein kinase A (P 5511)
Disclaimer
Please note that the pack size has been changed to align with the unit definition, while the number of phosphorylating units remain the same as before.
Inhibitor
Č. produktu
Popis
Stanovení ceny
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Osvědčení o analýze (COA)
Vyhledejte osvědčení Osvědčení o analýze (COA) zadáním čísla šarže/dávky těchto produktů. Čísla šarže a dávky lze nalézt na štítku produktu za slovy „Lot“ nebo „Batch“.
Již tento produkt vlastníte?
Dokumenty související s produkty, které jste v minulosti zakoupili, byly za účelem usnadnění shromážděny ve vaší Knihovně dokumentů.
Zákazníci si také prohlíželi
Christopher M Bocchiaro et al.
The Journal of neuroscience : the official journal of the Society for Neuroscience, 23(4), 1099-1103 (2003-02-25)
Plasticity underlying adaptive, long-term changes in breathing behavior is hypothesized to be attributable to the modulation of respiratory motoneurons by intracellular second-messenger cascades. In quiescent preparations, protein kinases, including cAMP-dependent protein kinase A (PKA), potentiate glutamatergic inputs. However, the dynamic
Petr G Vikhorev et al.
Cardiovascular research (2020-11-03)
Dilated cardiomyopathy (DCM) is associated with mutations in many genes encoding sarcomere proteins. Truncating mutations in the titin gene TTN are the most frequent. Proteomic and functional characterisations are required to elucidate the origin of the disease and the pathogenic
Protein kinase A activity and Hedgehog signaling pathway
Vitamins & Hormones, 88, 273-291 (2012)
J A Byun et al.
Science advances, 6(25), eabb1250-eabb1250 (2020-07-01)
The functional response of a signaling system to an allosteric stimulus often depends on subcellular conditions, a phenomenon known as pluripotent allostery. For example, a single allosteric modulator, Rp-cAMPS, of the prototypical protein kinase A (PKA) switches from antagonist to
E Wischmeyer et al.
Proceedings of the National Academy of Sciences of the United States of America, 93(12), 5819-5823 (1996-06-11)
Strongly rectifying IRK-type inwardly rectifying K+ channels are involved in the control of neuronal excitability in the mammalian brain. Whole-cell patch-clamp experiments show that cloned rat IRK1 (Kir 2.1) channels, when heterologously expressed in mammalian COS-7 cells, are inhibited following
Náš tým vědeckých pracovníků má zkušenosti ve všech oblastech výzkumu, včetně přírodních věd, materiálových věd, chemické syntézy, chromatografie, analytiky a mnoha dalších..
Obraťte se na technický servis.