8.52050
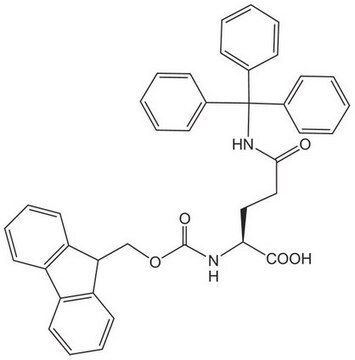
Fmoc-Trp(Boc)-OH
Novabiochem®
Synonyma:
Fmoc-Trp(Boc)-OH, N-α-Fmoc-N-in-t.-Boc-L-tryptophan
About This Item
Doporučené produkty
Quality Level
product line
Novabiochem®
assay
≥90.0% (acidimetric)
≥97.5% (HPLC)
≥98% (TLC)
form
powder
reaction suitability
reaction type: Fmoc solid-phase peptide synthesis
manufacturer/tradename
Novabiochem®
application(s)
peptide synthesis
functional group
Boc
Fmoc
storage temp.
15-25°C
InChI
1S/C31H30N2O6/c1-31(2,3)39-30(37)33-17-19(20-10-8-9-15-27(20)33)16-26(28(34)35)32-29(36)38-18-25-23-13-6-4-11-21(23)22-12-5-7-14-24(22)25/h4-15,17,25-26H,16,18H2,1-3H3,(H,32,36)(H,34,35)/t26-/m0/s1
InChI key
ADOHASQZJSJZBT-SANMLTNESA-N
Související kategorie
General description
The use of this N-in-Boc protected derivative overcomes most of the problems associated with the preparation of Trp containing-peptides by Fmoc SPPS [1]. Cleavage with TFA generates an N-in-carboxy indole which protects the Trp from alkylation [1,2,3] and sulfonation [1,4,5,6,7]. The N-in-carboxy group is removed under aqueous conditions during normal work-up of the peptide.
Associated Protocols and Technical Articles
Fmoc-amino acids for Peptide Production
Cleavage and Deprotection Protocols for Fmoc SPPS
Literature references
[1] P. White in ′Peptides, Chemistry & Biology, Proc. 12th American Peptide Symposium′, J. A. Smith & J. E. Rivier (Eds), ESCOM, Leiden, 1992, pp. 537.
[2] B. Riniker, et al. (1993) Tetrahedron, 49, 9307.
[3] T. Johnson, et al. (1993) J. Chem. Soc., Chem. Commun., 369.
[4] H. Choi, et al. (1993) Int. J. Peptide Protein Res., 42, 58.
[5] C. G. Fields, et al. (1993) Tetrahedron Lett., 34, 6661.
[6] T. Lescrinier, et al. (1995) Lett. Pept. Sci., 2, 225.
[7] M. Noda & M. Kiffe (1997) J. Peptide Res., 50, 329.
Application
- Highly active antibacterial ferrocenoylated or ruthenocenoylated Arg-Trp peptides can be discovered by an L-to-D substitution scan: Utilizes Fmoc-Trp(Boc)-OH in the synthesis of peptides designed to enhance antibacterial activity, demonstrating its role in the development of new antimicrobial agents (Albada et al., 2014).
- Nanoparticles generated from a tryptophan derivative: physical characterization and anti-cancer drug delivery: Explores the use of Fmoc-Trp(Boc)-OH in nanoparticle formation for targeted drug delivery systems, particularly in cancer therapy (Dube et al., 2017).
Linkage
Analysis Note
Appearance of substance (visual): powder
Colour index (0,5 M in DMF): ≤ 150 Hazen
Identity (IR): passes test
Enantiomeric purity: ≥ 99.7 % (a/a)
Purity (HPLC): ≥ 97.5 % (a/a)
Fmoc-ß-Ala-OH (HPLC): ≤ 0.3 % (a/a)
Fmoc-ß-Ala-Trp (Boc)-OH (HPLC): ≤ 0.1 % (a/a)
Fmoc-Trp(Boc)-Trp(Boc)-OH (HPLC): ≤ 0.1 % (a/a)
Fmoc-Trp-OH (HPLC): ≤ 1.0 % (a/a)
Assay free amino acid (HPLC): ≤ 0.2 %
Solubility (25 mmole in 50 ml DMF): clear soluble
Purity (TLC(011A)): ≥ 98 %
Purity (TLC(0811)): ≥ 98 %
Assay (acidimetric): ≥ 90.0 %
Water (K. F.): ≤ 2.0 %
Ethyl acetate (HS-GC): ≤ 2.5 %
Acetate (IC): ≤ 0.10 %
To see the solvent systems used for TLC of Novabiochem® products please click here.
Legal Information
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Osvědčení o analýze (COA)
Vyhledejte osvědčení Osvědčení o analýze (COA) zadáním čísla šarže/dávky těchto produktů. Čísla šarže a dávky lze nalézt na štítku produktu za slovy „Lot“ nebo „Batch“.
Již tento produkt vlastníte?
Dokumenty související s produkty, které jste v minulosti zakoupili, byly za účelem usnadnění shromážděny ve vaší Knihovně dokumentů.
Zákazníci si také prohlíželi
Související obsah
Purer Fmocs Means Purer Peptides
Náš tým vědeckých pracovníků má zkušenosti ve všech oblastech výzkumu, včetně přírodních věd, materiálových věd, chemické syntézy, chromatografie, analytiky a mnoha dalších..
Obraťte se na technický servis.