512125
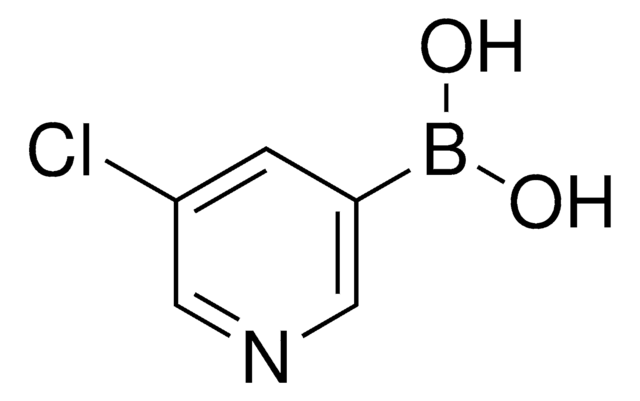
3-Pyridinylboronic acid
≥95.0%
Synonyma:
3-Pyridineboronic acid, 3-Pyridylboronic acid, Dihydroxy(3-pyridyl)borane, Pyridin-3-ylboronic acid
About This Item
Doporučené produkty
assay
≥95.0%
form
solid
mp
>300 °C (lit.)
SMILES string
OB(O)c1cccnc1
InChI
1S/C5H6BNO2/c8-6(9)5-2-1-3-7-4-5/h1-4,8-9H
InChI key
ABMYEXAYWZJVOV-UHFFFAOYSA-N
Související kategorie
Application
- Phosphine-free Suzuki-Miyaura cross-coupling reactions.
- Regioselective Suzuki-Miyaura coupling and tandem palladium-catalyzed intramolecular aminocarbonylation and annulation.
- N-arylation using copper acetylacetonate catalyst.
- Copper-mediated cyanation and regioselective cyanation of electron-rich benzenes.
It can also be used to prepare:
- New linear poly(phenylpyridyl) chains by Suzuki coupling.
- Oligopyridyl foldamers as mimics of a-helix twist.
- Many highly significant therapeutic enzymatic and kinase inhibitors and receptor antagonists.
- Pyridine substituted pyridinium N-(2′-azinyl)aminides by reacting with dibromo pyridinium aminides via Suzuki coupling reaction.
Other Notes
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Osvědčení o analýze (COA)
Vyhledejte osvědčení Osvědčení o analýze (COA) zadáním čísla šarže/dávky těchto produktů. Čísla šarže a dávky lze nalézt na štítku produktu za slovy „Lot“ nebo „Batch“.
Již tento produkt vlastníte?
Dokumenty související s produkty, které jste v minulosti zakoupili, byly za účelem usnadnění shromážděny ve vaší Knihovně dokumentů.
Zákazníci si také prohlíželi
Sortimentní položky
Suzuki-Miyaura cross-coupling reaction is extensively used in organic chemistry, polymer science, and pharmaceutical industries.
Náš tým vědeckých pracovníků má zkušenosti ve všech oblastech výzkumu, včetně přírodních věd, materiálových věd, chemické syntézy, chromatografie, analytiky a mnoha dalších..
Obraťte se na technický servis.