37760
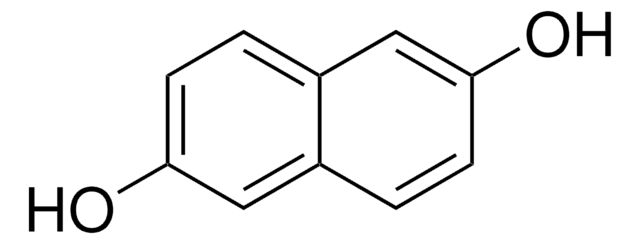
2,3-Dihydroxynaphthalene
≥98.0% (HPLC)
Synonyma:
2,3-Naphthalenediol
Přihlásitk zobrazení cen stanovených pro organizaci a smluvních cen
About This Item
Lineární vzorec:
C10H6(OH)2
Číslo CAS:
Molekulová hmotnost:
160.17
Beilstein/REAXYS Number:
742375
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Doporučené produkty
Quality Level
assay
≥98.0% (HPLC)
sublimation residue
≤1%
mp
161-165 °C (lit.)
162-164 °C
SMILES string
Oc1cc2ccccc2cc1O
InChI
1S/C10H8O2/c11-9-5-7-3-1-2-4-8(7)6-10(9)12/h1-6,11-12H
InChI key
JRNGUTKWMSBIBF-UHFFFAOYSA-N
Gene Information
human ... BAD(572)
Hledáte podobné produkty? Navštivte Průvodce porovnáváním produktů
Související kategorie
General description
2,3-Dihydroxynaphthalene is a polyhydroxy phenol. It is an aromatic dihydroxy compound having hydroxyl groups at ortho positions. Its reaction with molybdenum(VI) complexes has been reported. The asymmetric oxidative coupling polymerization of 2,3-dihydroxynaphthalene using the Cu(I)-bisoxazoline complex as catalyst has been reported to afford poly(2,3-dihydroxy-1,4-naphthylene), having a continuous 1,1′-bi-2-naphthol main chain structure. The nitrodisplacement reaction between nitrophthalodinitriles and 2,3-dihydroxynaphthalene has been investigated.
Application
2,3-Dihydroxynaphthalene may be used in the following studies:
- Construction of dinaphtho[2,1-b;2′,3′-d]furan-6-ol, via dehydration reaction in the presence of strong acid.
- As fused ring catecholate type ligand for the surface modification of nanocrystalline TiO2 particles.
- As adsorptive and competing ligand during the chemical speciation of iron in seawater by cathodic stripping voltammetry.
- Synthesis of cyclotriphosphazene derivatives, used as non-halogen flame retardants
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
347.0 °F
flash_point_c
175 °C
ppe
dust mask type N95 (US), Eyeshields, Gloves
Vyberte jednu z posledních verzí:
Již tento produkt vlastníte?
Dokumenty související s produkty, které jste v minulosti zakoupili, byly za účelem usnadnění shromážděny ve vaší Knihovně dokumentů.
Zákazníci si také prohlíželi
Copper (I)-catalyzed asymmetric oxidative coupling polymerization of 2, 3-dihydroxynaphthalene using bisoxazoline ligands.
Habaue S, et al.
Macromolecules, 36(8), 2604-2608 (2003)
Application of cyclophosphazene derivatives as flame retardants for ABS.
Shin YJ, et al.
Journal of Industrial and Engineering Chemistry (Amsterdam, Netherlands), 16(3), 364-367 (2010)
Synthesis of bis (ether anhydride) s for poly (ether imide) s having 1, 2-linked units by nitrodisplacement with catechol derivatives.
Eastmond GC and Paprotny J.
Macromolecules, 28(7), 2140-2146 (1995)
Tatjana D Savić et al.
Nanoscale, 4(5), 1612-1619 (2012-02-09)
Surface modification of nanocrystalline TiO(2) particles (45 Å) with catecholate-type ligands consisting of an extended aromatic ring system, i.e., 2,3-dihydroxynaphthalene and anthrarobin, was found to alter the optical properties of the nanoparticles in a similar way to modification with catechol.
Kentaro Nakanishi et al.
The Journal of organic chemistry, 79(6), 2625-2631 (2014-02-26)
The construction of dinaphtho[2,1-b;2',3'-d]furan-6-ol was developed via a dehydration reaction involving two molecules of 2,3-dihydroxynaphthalene in the presence of a strong acid. Starting from the dinaphthofuran, a variety of butterfly shaped derivatives were synthesized. The optical properties of these compounds
Náš tým vědeckých pracovníků má zkušenosti ve všech oblastech výzkumu, včetně přírodních věd, materiálových věd, chemické syntézy, chromatografie, analytiky a mnoha dalších..
Obraťte se na technický servis.