About This Item
Lineární vzorec:
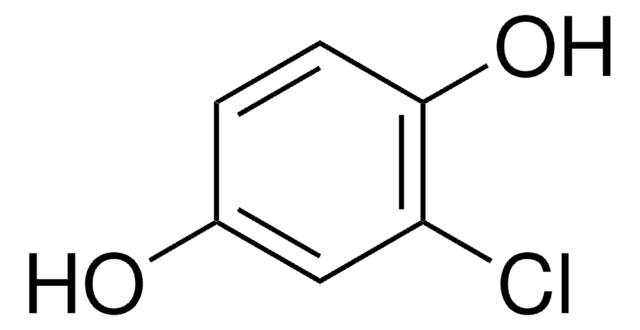
BrC6H3(OH)2
Číslo CAS:
Molekulová hmotnost:
189.01
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Doporučené produkty
assay
97%
mp
112-116 °C (lit.)
functional group
bromo
SMILES string
Oc1ccc(O)c(Br)c1
InChI
1S/C6H5BrO2/c7-5-3-4(8)1-2-6(5)9/h1-3,8-9H
InChI key
REFDOIWRJDGBHY-UHFFFAOYSA-N
Související kategorie
Application
Bromohydroquinone was used in the synthesis of Π-conjugated polymers composed of alkyl carbazole/dialkoxyphenylene and squaraine units via Sonogashira cross-coupling reactions. It was used in the preparation of 2-bromobenzoquinone.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Vyberte jednu z posledních verzí:
Osvědčení o analýze (COA)
Lot/Batch Number
Nevidíte správnou verzi?
Potřebujete-li konkrétní verzi, můžete vyhledat daný certifikát podle čísla dávky nebo čísla šarže.
Již tento produkt vlastníte?
Dokumenty související s produkty, které jste v minulosti zakoupili, byly za účelem usnadnění shromážděny ve vaší Knihovně dokumentů.
Zákazníci si také prohlíželi
S S Lau et al.
The Journal of pharmacology and experimental therapeutics, 230(2), 360-366 (1984-08-01)
2-Bromohydroquinone was identified as a metabolite of both bromobenzene and o-bromophenol in the rat in vivo and in vitro. Identification was based on high-pressure liquid chromatography and gas chromatography-mass spectrometry. Formation of 2-bromohydroquinone by rat liver microsomes from both bromobenzene
T J Monks et al.
Toxicology and applied pharmacology, 103(3), 557-563 (1990-05-01)
Glutathione (GSH) conjugates of 2-bromohydroquinone are more difficult to oxidize than the parent hydroquinone. Hydrolysis catalyzed by gamma-glutamyl transpeptidase (gamma-GT), however, results in the formation of the corresponding cysteine conjugate which is more readily oxidized than the parent hydroquinone. N-Acetylation
S S Lau et al.
Drug metabolism and disposition: the biological fate of chemicals, 15(6), 801-807 (1987-11-01)
Homogenates from rat renal papillae, a rich source of the prostaglandin (PG) H synthase system (PHS), metabolized [14C]2-bromohydroquinone, in the presence of arachidonic acid, to products which are covalently bound to protein. The co-oxidation of 2-bromohydroquinone caused a concentration-dependent stimulation
S S Lau et al.
Toxicology and applied pharmacology, 103(1), 121-132 (1990-03-15)
We have previously shown that the renal necrosis observed after 2-bromohydroquinone (2-BrHQ) administration to rats is probably caused by the formation of 2-Br-(diglutathion-S-yl)HQ (2-Br-[diGSyl]HQ), since injection of this conjugate caused severe proximal tubular necrosis. In the present study we report
Hetero Diels-Alder Reactions of 1-Acetylamino-and 1-Dimethylamino-1-azadienes with Benzoquinones.
Perez JM, et al.
Tetrahedron, 56(11), 1561-1567 (2000)
Náš tým vědeckých pracovníků má zkušenosti ve všech oblastech výzkumu, včetně přírodních věd, materiálových věd, chemické syntézy, chromatografie, analytiky a mnoha dalších..
Obraťte se na technický servis.