Descrição geral
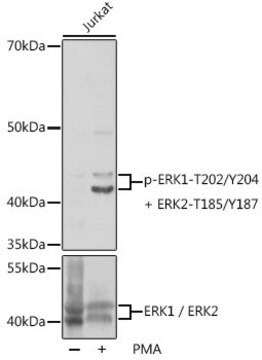
Extracellular Signal-Regulated Kinases (ERKs) are members of mitogen-activated protein kinase superfamily (MAPK). MAPK cascade is an evolutionary conserved module that mediates the signaling from various extracellular stimuli to the nucleus. The well-characterized ERK module is activated in response to stimuli such as cytokines, growth factors, osmotic shock, or UV irradiation. ERKs regulate transcription, cell cycle, differentiation, learning, and memory through signal transduction in the cytoplasm and the nucleus. ERK1 and 2 phosphorylate microtubule-associated protein-2 (MAP2), myelin basic protein (MBP), and ELK-1. They may promote entry in the cell cycle. The ERK cascade connects to G proteins through a multitude of distinct signal transduction pathways. ERK1 (p44) and ERK2 (p42) require the dual phosphorylation in the catalytic kinase domain by MEKs for their full activity. ERK1 is phosphorylated on Tyr204 and Thr202, and ERK2 on Tyr187 and Thr185.
Mitogen-activated protein kinase (MAPK) superfamily of enzymes is involved in widespread signalling pathways. Members of this family include the ERK1/2 (extracellular signal-regulated protein kinase, also termed p42/p44 MAPK), JNK and p38 MAPK subfamilies. These are the terminal enzymes in a signalling cascade where each kinase phosphorylates and activates the next member in the sequence. Phosphorylation of both tyrosine and threonine is essential for the full activation of all MAPKs. Several kinases participate in activation of the ERK cascade. This cascade is initiated by the small G protein Ras, which upon stimulation causes activation Raf1 kinase. Raf1 continues the transmission by activating MEK. Activated MEK appears to be the only kinase capable of specifically phosphorylating and activating ERK. ERK1 (p44) and ERK2 (p42) require the dual phosphorylation in the catalytic kinase domain by MEKs for their full activity. ERK1 is phosphorylated on Tyr204 and Thr202, and ERK2 on Tyr187 and Thr185. ERK1 and 2 may also undergo autophosphorylation on these residues. ERK appears to be an important regulatory molecule, which by can phosphorylate regulatory targets in the cytosol (phospholipase A2, PLA2), translocated into and phosphorylate substrates in the nucleus (ELK1). The activation of ERK cascade mediates and regulates the signal transduction pathways in response to stress, mitogenic signals and is important in development and differentiation, learning, memory and survival.
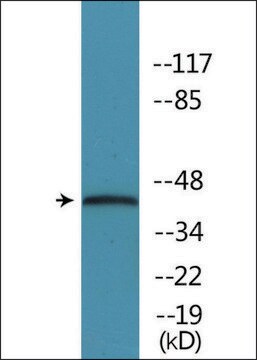
This antibody recognises endogenous active forms of ERK1/2 (44/42 kDa) in human, mouse, rat and chick embryo.
Especificidade
Rabbit polyclonal anti-phospho-ERK1 (pThr202/pTyr204) and ERK2 (pThr185/pTyr187) antibody recognizes endogenous active forms of ERK1 & 2 (44 kDa and 42 kDa, respectively) in a variety of cell types, including human, mouse, rat and chick embryo.
Imunogênio
synthetic phosphopeptide derived from the region of human ERK1 and 2 that contain threonine 202/185 and tyrosine 204/187 respectively.
Aplicação
A working dilution of 1:1000 is recommended for detection of ERK1 (phosphorylated at Thr202/185) and ERK2 (phosphorylated at Tyr204/187) in PC12 cells. The antibody is also suitable for immunocytochemistry and immunohistochemistry in human midbrain sections at a working dilution of 1:500 and 1:2000, respectively.
Rabbit polyclonal anti-phospho-ERK1 (pThr202/pTyr204) and ERK2 (pThr185/pTyr187) antibody may be used in immunoblotting and immunostaining applications. It is used to detect the presence of activated ERK1 and ERK2.
forma física
Solution in Dulbecco′s with 50% glycerol, BSA and sodium azide.
Exoneração de responsabilidade
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.