The CMR (Carcinogenic, Mutagenic, or Reproductive toxic) classification related to this product is affected by both iron-dextran and phenol. Iron-dextran may potentially be a human carcinogen, while phenol is associated with the risk of causing genetic defects. For detailed information, please refer to Section 11.2 in the Safety Data Sheet (SDS) available at the following link: https://www.sigmaaldrich.com/sds/sigma/d8517?userType=anonymous
D8517
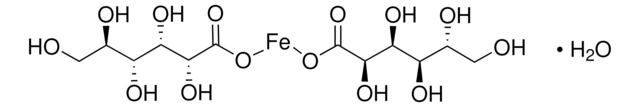
Iron–Dextran
95-105 mg/mL (Iron content), solution
Sinônimo(s):
Ferric hydroxide dextran complex
Selecione um tamanho
Selecione um tamanho
About This Item
Produtos recomendados
fonte biológica
synthetic
Nível de qualidade
esterilidade
aseptically filled
Ensaio
95-105 mg/mL (Iron content)
Formulário
solution
contém
0.5% phenol as preservative
composição
Iron, ~100 mg/mL
cor
brown
faixa de pH útil
4 - 6.5
temperatura de armazenamento
room temp
cadeia de caracteres SMILES
[Fe].[S](=O)(=O)(O)O
InChI
1S/Fe.H2O4S/c;1-5(2,3)4/h;(H2,1,2,3,4)
chave InChI
MVZXTUSAYBWAAM-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Aplicação
Outras notas
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Carc. 1B - Skin Sens. 1
Código de classe de armazenamento
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Escolha uma das versões mais recentes:
Certificados de análise (COA)
Não está vendo a versão correta?
Se precisar de uma versão específica, você pode procurar um certificado específico pelo número do lote ou da remessa.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
-
Is the CMR danger related to the presence of Phenol as a preservative or other components ? and/or Iron-dextran itself ?
1 answer-
Helpful?
-
-
How can I determine the shelf life / expiration / retest date of this product?
1 answer-
If this product has an expiration or retest date, it will be shown on the Certificate of Analysis (COA, CofA). If there is no retest or expiration date listed on the product's COA, we do not have suitable stability data to determine a shelf life. For these products, the only date on the COA will be the release date; a retest, expiration, or use-by-date will not be displayed.
For all products, we recommend handling per defined conditions as printed in our product literature and website product descriptions. We recommend that products should be routinely inspected by customers to ensure they perform as expected.
For products without retest or expiration dates, our standard warranty of 1 year from the date of shipment is applicable.
For more information, please refer to the Product Dating Information document: https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/marketing/global/documents/449/386/product-dating-information-mk.pdfHelpful?
-
-
How is shipping temperature determined? And how is it related to the product storage temperature?
1 answer-
Products may be shipped at a different temperature than the recommended long-term storage temperature. If the product quality is sensitive to short-term exposure to conditions other than the recommended long-term storage, it will be shipped on wet or dry-ice. If the product quality is NOT affected by short-term exposure to conditions other than the recommended long-term storage, it will be shipped at ambient temperature. As shipping routes are configured for minimum transit times, shipping at ambient temperature helps control shipping costs for our customers. For more information, please refer to the Storage and Transport Conditions document: https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/marketing/global/documents/316/622/storage-transport-conditions-mk.pdf
Helpful?
-
-
I am interested in the dextran concentration and the molcular weight of the dextran of the iron dextran solution (D8517)
1 answer-
The detran concentration is not determined for this product. The iron particles are coated with dextran by Ven der Waals forces, allowing them to remain in solution. The dextran used in the preparation is partically hydrolyzed and has an approximate molecular weight of 5000 daltons.
Helpful?
-
-
Provide the information about concentration for this product and further solubility.
1 answer-
The product is provided as a 100 mg iron/ml solution in water, containing 0.5% phenol as a preservative. It can be further diluted in water or buffer.
Helpful?
-
Active Filters
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica