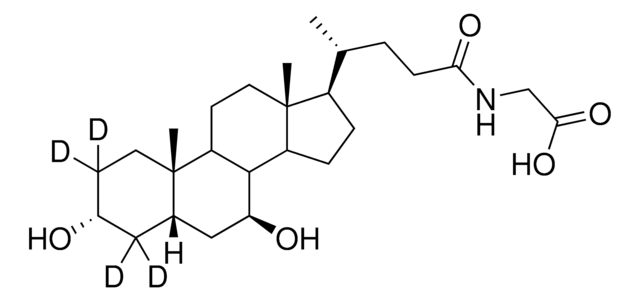
06863
Glycoursodeoxycholic acid
≥96.0% (TLC)
Sinônimo(s):
N-(3α,7β-Dihydroxy-5β-cholan-24-oyl)glycine, N-[(3α,5β,7β)-3,7-Dihydroxy-24-oxocholan-24-yl]glycine, GUDCA, Glycylursodeoxycholic acid, Ursodeoxycholylglycine
About This Item
Produtos recomendados
fonte biológica
synthetic
Ensaio
≥96.0% (TLC)
forma
powder
grupo funcional
carboxylic acid
cadeia de caracteres SMILES
[H][C@@]12[C@]([C@](CC[C@@H](O)C3)(C)[C@]3([H])C[C@@H]2O)([H])CC[C@@]4(C)[C@@]1([H])CC[C@]4([H])[C@]([H])(C)CCC(NCC(O)=O)=O
InChI
1S/C26H43NO5/c1-15(4-7-22(30)27-14-23(31)32)18-5-6-19-24-20(9-11-26(18,19)3)25(2)10-8-17(28)12-16(25)13-21(24)29/h15-21,24,28-29H,4-14H2,1-3H3,(H,27,30)(H,31,32)/t15-,16+,17-,18-,19+,20+,21+,24+,25+,26-/m1/s1
chave InChI
GHCZAUBVMUEKKP-XROMFQGDSA-N
Categorias relacionadas
Aplicação
- Glycoursodeoxycholic Acid Alleviates Arterial Thrombosis via Suppressing Diacylglycerol Kinases Activity in Platelet.: Highlights the therapeutic potential of Glycoursodeoxycholic acid in alleviating arterial thrombosis by inhibiting diacylglycerol kinase activity in platelets (Yang et al., 2024).
Ações bioquímicas/fisiológicas
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Certificados de análise (COA)
Busque Certificados de análise (COA) digitando o Número do Lote do produto. Os números de lote e remessa podem ser encontrados no rótulo de um produto após a palavra “Lot” ou “Batch”.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Protocolos
Investigate bile acid roles in gut hormone profiles and glycemic control, vital for clinical labs exploring potential mechanisms.
Conteúdo relacionado
Bile Acids (BA) are synthesized in the liver and play important roles in cholesterol homeostasis, absorption of vitamins and lipids, and various key metabolic processes.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica