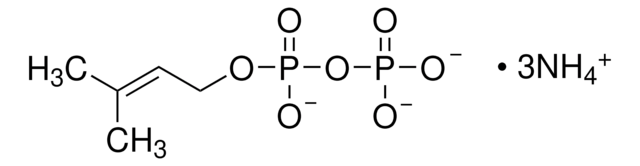
E6376
Erythromycin
potency: ≥850 μg per mg
Sinônimo(s):
Erythromycin A
About This Item
Produtos recomendados
fonte biológica
Streptomyces erythreus
Nível de qualidade
forma
powder
potência
≥850 μg per mg
cor
white
solubilidade
ethanol: 50 mg/mL, clear to slightly hazy, colorless to faintly yellow
espectro de atividade do antibiótico
Gram-negative bacteria
Gram-positive bacteria
Modo de ação
protein synthesis | interferes
cadeia de caracteres SMILES
CC[C@H]1OC(=O)[C@H](C)[C@@H](O[C@H]2C[C@@](C)(OC)[C@@H](O)[C@H](C)O2)[C@H](C)[C@@H](O[C@@H]3O[C@H](C)C[C@@H]([C@H]3O)N(C)C)[C@](C)(O)C[C@@H](C)C(=O)[C@H](C)[C@@H](O)[C@]1(C)O
InChI
1S/C37H67NO13/c1-14-25-37(10,45)30(41)20(4)27(39)18(2)16-35(8,44)32(51-34-28(40)24(38(11)12)15-19(3)47-34)21(5)29(22(6)33(43)49-25)50-26-17-36(9,46-13)31(42)23(7)48-26/h18-26,28-32,34,40-42,44-45H,14-17H2,1-13H3/t18-,19-,20+,21+,22-,23+,24+,25-,26+,28-,29+,30-,31+,32-,34+,35-,36-,37-/m1/s1
chave InChI
ULGZDMOVFRHVEP-RWJQBGPGSA-N
Informações sobre genes
human ... ABCB1(5243) , CYP3A4(1576) , MLNR(2862)
mouse ... Abcb1a(18671) , Abcb1b(18669)
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Descrição geral
Aplicação
Erythromycin is an antibiotic produced by growth of certain strains of Streptomyces erythreus. This product is composed largely of erythromycin A with small amounts of erythromycins B and C and is recommended for concentration at 100 mg/L. Concentrations between 50 and 200 mg/L have also proven effective in controlling bacterial growth. Erythromycin has been used as a motilin receptor agonist, to block respiratory glycoconjugate secretion in human airways in vitro, and for selecting plasmid-cured and recombinant lactococcus lactis MG1363 strains.
Ações bioquímicas/fisiológicas
Antimicrobial Spectrum: This product acts against both gram-negative and gram-positive bacteria.
Atenção
Nota de preparo
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Artigos
Antibiotics targeting bacterial ribosomes disrupt protein synthesis, a key process in bacterial growth inhibition.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica