15450
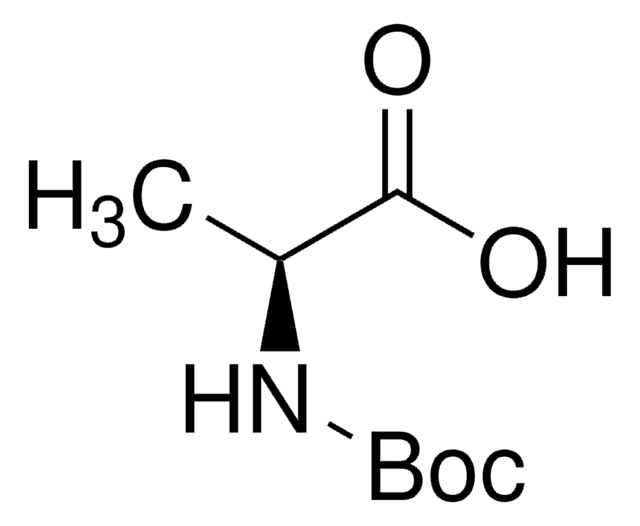
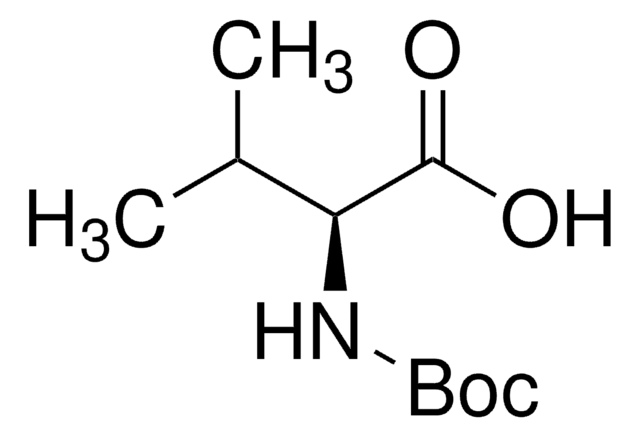
Boc-Leu-OH hydrate
≥99.0% (HPLC)
Sinônimo(s):
Boc-L-leucine
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula linear:
(CH3)2CHCH2CH(COOH)NHCOOC(CH3)3 xH2O
Peso molecular:
231.29 (anhydrous basis)
Número CE:
Número MDL:
Código UNSPSC:
12352209
eCl@ss:
32160406
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
≥99.0% (HPLC)
forma
solid
atividade óptica
[α]20/D −25±0.5°, c = 2% in acetic acid
adequação da reação
reaction type: Boc solid-phase peptide synthesis
reaction type: C-H Activation
reagent type: ligand
reaction type: Peptide Synthesis
pf
85-90 °C
aplicação(ões)
peptide synthesis
grupo funcional
amine
carboxylic acid
cadeia de caracteres SMILES
CC(C)C[C@H](NC(OC(C)(C)C)=O)C(O)=O
Categorias relacionadas
Aplicação
Boc-Leu-OH (Boc-L-leucine) was used in the synthesis of a potent cytotoxin, PM-94128.
Boc-protected leucine (Boc-Leu-OH) can be used to generate combinatorial peptide libraries and also to synthesize peptide models to study structure-activity relationships.
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Multicyclic polypeptide model compounds. 1. Synthesis of a tricyclic amphiphilic. alpha.-helical peptide using an oxime resin, segment-condensation approach.
Osapay G, et al.
Journal of the American Chemical Society, 112(16), 6046-6051 (1990)
Screening of mixture combinatorial libraries for chiral selectors: a reciprocal chromatographic approach using enantiomeric libraries.
Wu Y, et al.
Analytical Chemistry, 71(9), 1688-1691 (1999)
Masaru Enomoto et al.
The Journal of organic chemistry, 74(19), 7566-7569 (2009-09-03)
The enantioselective total synthesis of PM-94128, a potent cytotoxin of microbial origin, was accomplished by a concise nine-step sequence of reactions in 14% overall yield from N-Boc-l-leucine. The synthesis of Y-05460M-A, a one-carbon lower homologue of PM-94128, was also achieved
The synthesis and screening of a combinatorial peptide library for affinity ligands for glycosylated haemoglobin1.
Chen B, et al.
Biosensors And Bioelectronics, 13(7-8), 779-785 (1998)
Hyun-Woong Cho et al.
PloS one, 14(6), e0217745-e0217745 (2019-06-21)
The aim of this study was to investigate the short-term efficacy and safety of Poly-gamma-glutamic acid (γ-PGA) and the immunologic changes in patients with CIN 1. Participants were randomly assigned to one of two groups and orally treated with placebo
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica