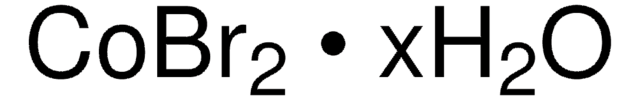03858
Dimethylglyoxime
≥97.0% (TLC)
Sinônimo(s):
dmgH2, 2,3-Butanedione dioxime, Diacetyldioxime
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula linear:
CH3C(=NOH)C(=NOH)CH3
Número CAS:
Peso molecular:
116.12
Beilstein:
506731
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
≥97.0% (TLC)
forma
powder
pf
240-241 °C (lit.)
cadeia de caracteres SMILES
CC(=N/O)\C(C)=N\O
InChI
1S/C4H8N2O2/c1-3(5-7)4(2)6-8/h7-8H,1-2H3/b5-3+,6-4+
chave InChI
JGUQDUKBUKFFRO-GGWOSOGESA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Aplicação
Dimethylglyoxime can be used:
- As a ligand for the synthesis of cobaloxime complexes which are used as electrocatalysts for the production of hydrogen by electroreduction of protons.
- As a precipitant for the synthesis of NiO and CuI nanoparticles.P
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Flam. Sol. 2
Código de classe de armazenamento
4.1B - Flammable solid hazardous materials
Classe de risco de água (WGK)
WGK 3
Equipamento de proteção individual
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Choose from one of the most recent versions:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Proton electroreduction catalyzed by cobaloximes: Functional models for hydrogenases.
Razavet M, et al.
Inorganic Chemistry, 44(13), 4786-4795 (2005)
Synthesis and characteristics of NiO nanoparticles by thermal decomposition of nickel dimethylglyoximate rods.
Li X, et al.
Solid State Communications, 137(11), 581-584 (2006)
Hua-Jian Xu et al.
Organic & biomolecular chemistry, 10(13), 2562-2568 (2012-02-23)
CuI nanoparticles efficiently catalyzed the C-S cross coupling of aryl and alkyl thiols with aryl halides in the absence of ligands on water under mild conditions. A wide range of diaryl sulfides and aryl alkyl sulfides are synthesized in good
Jandee Kim et al.
Langmuir : the ACS journal of surfaces and colloids, 27(23), 14638-14646 (2011-10-18)
Dimethylglyoxime (DMG) adsorbed on Au(111) was investigated using electrochemical scanning tunneling microscopy (STM) and X-ray photoelectron spectroscopy (XPS). STM experiments revealed three different structures of adsorbed DMG at open circuit potential (~0.07 V versus Ag/AgCl): (2√3×2√3)R30°-α, (2√3×4√3)R30°-β, and (2√3×4√3)R30°-γ. The
Carsten R Hamann et al.
Contact dermatitis, 68(1), 15-22 (2012-12-12)
Nickel is widely used in coins; nickel may cause contact allergy and allergic contact dermatitis in those who handle them. To investigate alloy use, coin composition and nickel and cobalt release for a worldwide selection of currently circulating coins. Eight
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica