513997
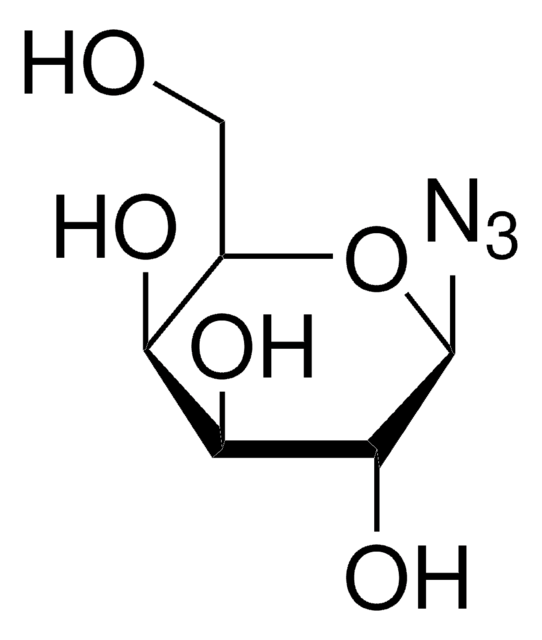
1-Azido-1-deoxy-β-D-glucopyranoside tetraacetate
Sinônimo(s):
NSC 272456
About This Item
Produtos recomendados
forma
solid
atividade óptica
[α]/D -29°, c = 1% in H2O
[α]/D -30°, c = 1% in chloroform
adequação da reação
reaction type: click chemistry
pf
127-131 °C (lit.)
cadeia de caracteres SMILES
CC(=O)OC[C@H]1O[C@@H](N=[N+]=[N-])[C@H](OC(C)=O)[C@@H](OC(C)=O)[C@H]1OC(C)=O
InChI
1S/C14H19N3O9/c1-6(18)22-5-10-11(23-7(2)19)12(24-8(3)20)13(25-9(4)21)14(26-10)16-17-15/h10-14H,5H2,1-4H3/t10-,11+,12+,13-,14-/m1/s1
chave InChI
NHNYHKRWHCWHAJ-MBJXGIAVSA-N
Aplicação
- 1,2,3-Triazole-boron dipyrromethenes (BODIPYs) containing glucose groups via Cu(I)-catalyzed azide–alkyne ″click″ cycloaddition reaction conditions.
- 1-(β-D-glycosyl)-5-benzenesulfonamide-1,2,3-triazole derivatives by ruthenium-catalyzed azide-alkyne cycloaddition reactions.
- 2,3,4,6-Tetra-O-acetyl-β-D-glucopyranosylamine by palladium catalyzed hydrogenation reaction.
- Glycoside annulated dihydropyrimidinone derivatives by one-pot five-component condensation reaction with tert-butyl β-ketoester, arylaldehyde, urea and propargyl alcohol.
- Synthesis of Protein Tyrosine Phosphatase 1B inhibitor
- Synthesis of glycoconjugate carbonic anhydrase inhibitors by ruthenium-catalyzed azide-alkyne 1,3-dipolar cycloaddition
- Synthesis of variously coupled conjugates of D-glucose via click chemistry for inhibition of glycogen phosphorylase
- Hydrogenation reactions
- Preparation of posttranslationally modified peptides efficiently mimicking neoantigens in relation to autoimmune disease
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Escolha uma das versões mais recentes:
Certificados de análise (COA)
Não está vendo a versão correta?
Se precisar de uma versão específica, você pode procurar um certificado específico pelo número do lote ou da remessa.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica