126233
4,6-Dihydroxy-5-nitropyrimidine
95%
Sinônimo(s):
5-Nitro-4,6-pyrimidinediol
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C4H3N3O4
Número CAS:
Peso molecular:
157.08
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
Produtos recomendados
Ensaio
95%
forma
solid
pf
>300 °C (lit.)
grupo funcional
nitro
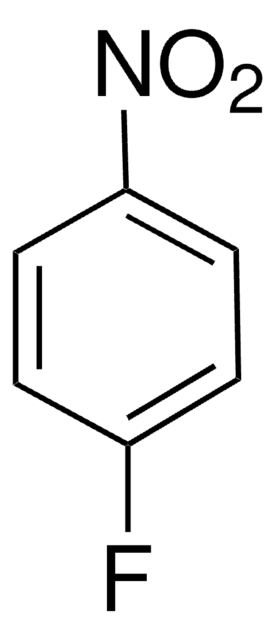
cadeia de caracteres SMILES
Oc1ncnc(O)c1[N+]([O-])=O
InChI
1S/C4H3N3O4/c8-3-2(7(10)11)4(9)6-1-5-3/h1H,(H2,5,6,8,9)
chave InChI
ABTLZAVJDRUDNG-UHFFFAOYSA-N
Ações bioquímicas/fisiológicas
4, 6-dihydroxy-5-nitropyrimidine is an inhibitor of thymidine phosphorylase activity. It is also a potent and selective inhibitor of 4-nitrophenol glucuronidation.
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
E Miszczak-Zaborska et al.
Zeitschrift fur Naturforschung. C, Journal of biosciences, 52(9-10), 670-675 (1997-11-28)
Partially purified samples of thymidine phosphorylase were obtained from four preparations of human uterine leiomyomas and uteri using the method of Yoshimura et al. (1990), Biochim. Biophys. Acta 1034, 107-113. Among the studied twelve pyrimidine derivatives, 5-bromouracil, 5-nitrouracil, 5-fluorouracil, 6-aminouracil
Z Naydenova et al.
Comparative biochemistry and physiology. Part C, Pharmacology, toxicology & endocrinology, 112(3), 321-325 (1995-11-01)
Thirty-one differently substituted pyrimidine bases were tested for their inhibitory effect on the glucuronidation of 4-nitrophenol and phenolphthalein by rat liver microsomes. 5-Nitrouracil (compound 1) and its isomer 4,6-dihydroxy-5-nitropyrimidine (compound 2) were the most potent and selective inhibitors of 4-nitrophenol
Andrew C Kotze et al.
Antimicrobial agents and chemotherapy, 58(12), 7475-7483 (2014-10-08)
We used an enzyme induction approach to study the role of detoxification enzymes in the interaction of the anthelmintic compound naphthalophos with Haemonchus contortus larvae. Larvae were treated with the barbiturate phenobarbital, which is known to induce the activity of
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica