E4906
Enterokinase from bovine intestine
BioUltra, recombinant, expressed in E. coli, ≥20 units/mg protein, ≥95% (SDS-PAGE)
Synonym(e):
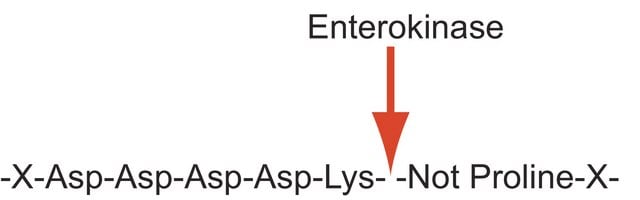
Enteropeptidase
About This Item
Empfohlene Produkte
Rekombinant
expressed in E. coli
Produktlinie
BioUltra
Assay
≥95% (SDS-PAGE)
Form
solution
Spezifische Aktivität
≥20 units/mg protein
Mol-Gew.
150 kDa
Verpackung
vial of ~0.2 unit
Konzentration
≥0.1 mg/mL
Versandbedingung
wet ice
Lagertemp.
−20°C
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Anwendung
Adjust the concentration of the fusion protein to 1.5 mg/ml and a pH between 7.0-8.0 with 500 mM Tris-HCl, pH 8.0, 2.0 mM CaCl2, and 1% Tween® 20
Add enterokinase to fusion protein solution at a ratio of ~ 0.02 units per 1 mg fusion protein and mix
Incubate reaction mixture at ~25 °C for 16 hours
Biochem./physiol. Wirkung
Physikalische Eigenschaften
Einheitendefinition
Physikalische Form
Rechtliche Hinweise
Lagerklassenschlüssel
10 - Combustible liquids
WGK
WGK 1
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.