70050
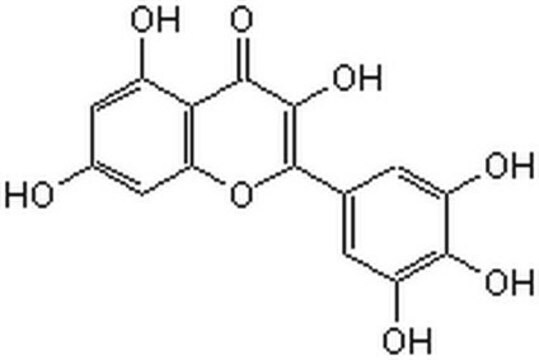
Myricetin
≥96.0% (HPLC)
Synonym(e):
3,3′,4′,5,5′,7-Hexahydroxy-flavon, Cannabiscetin, Myricetol
About This Item
Empfohlene Produkte
Qualitätsniveau
Assay
≥96.0% (HPLC)
Form
powder
mp (Schmelzpunkt)
≥300 °C
>300 °C (lit.)
Löslichkeit
ethanol: 10 mg/mL, clear to very faintly turbid, yellow to very deep greenish-yellow
Anwendung(en)
metabolomics
vitamins, nutraceuticals, and natural products
SMILES String
Oc1cc(O)c2C(=O)C(O)=C(Oc2c1)c3cc(O)c(O)c(O)c3
InChI
1S/C15H10O8/c16-6-3-7(17)11-10(4-6)23-15(14(22)13(11)21)5-1-8(18)12(20)9(19)2-5/h1-4,16-20,22H
InChIKey
IKMDFBPHZNJCSN-UHFFFAOYSA-N
Angaben zum Gen
human ... CYP1A2(1544)
mouse ... Hexa(15211)
rat ... Il4(287287) , Tnf(24835)
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Verwandte Kategorien
Allgemeine Beschreibung
Anwendung
- to study its preventive effect as an antioxidant on noise-induced hearing loss (NIHL) in rats
- as a flavonoid compound to test antiviral activity of Bourbon virus (BRBV) and in inhibition of RNA-dependent RNA polymerase (RdRP)
- to study its effect as a treatment on biofilms of Streptococcus mutans and Candida albicans
- as a reference standard for the quantification of phenolic compounds from Juniperus species
Biochem./physiol. Wirkung
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves, type N95 (US)
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Artikel
Antioxidants protect biological systems from oxidative damage produced by oxygen-containing free radicals and from redoxactive transition metal ions such as iron, copper, and cadmium.
Protokolle
Protocol for HPLC Analysis of Flavonoids on Ascentis® RP-Amide
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.