8.02954
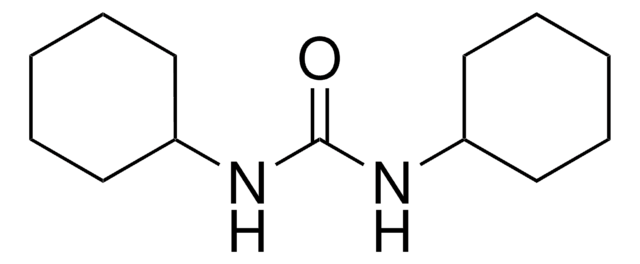
N,N′-Dicyclohexylcarbodiimide
for peptide synthesis
Synonym(e):
DCC
About This Item
Empfohlene Produkte
product name
N,N′-Dicyclohexylcarbodiimide, for synthesis
Qualitätsniveau
Form
solid
Wirksamkeit
1110 mg/kg LD50, oral (Rat)
71 mg/kg LD50, skin (Rat)
Eignung der Reaktion
reaction type: Coupling Reactions
bp
148-152 °C/15 hPa
mp (Schmelzpunkt)
35-36 °C
Übergangstemp.
flash point 113 °C
Dichte
0.95 g/cm3 at 40 °C
Schüttdichte
920 kg/m3
Anwendung(en)
peptide synthesis
Lagertemp.
2-30°C
InChI
1S/C13H22N2/c1-3-7-12(8-4-1)14-11-15-13-9-5-2-6-10-13/h12-13H,1-10H2
InChIKey
QOSSAOTZNIDXMA-UHFFFAOYSA-N
Verwandte Kategorien
Allgemeine Beschreibung
Anwendung
- The synthesis of optically pure N-acyl-N,N′-dicyclohexylureas.
- The activation of the carboxylic acid groups in aromatic carboxylic acids to facilitates their reaction with (N-isocyanimino)trifluoroacetamide to form the corresponding 1,3,4-oxadiazole derivatives.
- The synthesis of poly (vinyl alcohol-co-vinyl levulinate) copolymers for use in biomedical applications.
Signalwort
Danger
H-Sätze
Gefahreneinstufungen
Acute Tox. 3 Dermal - Acute Tox. 4 Oral - Eye Dam. 1 - Skin Sens. 1
Lagerklassenschlüssel
6.1D - Non-combustible, acute toxic Cat.3 / toxic hazardous materials or hazardous materials causing chronic effects
WGK
WGK 3
Flammpunkt (°F)
235.4 °F - closed cup
Flammpunkt (°C)
113 °C - closed cup
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.