推薦產品
等級
certified reference material
pharmaceutical secondary standard
品質等級
agency
traceable to BP 363
traceable to Ph. Eur. Y0000513
traceable to USP 1288500
API 家族
gemfibrozil
CofA
current certificate can be downloaded
技術
HPLC: suitable
gas chromatography (GC): suitable
應用
pharmaceutical (small molecule)
格式
neat
儲存溫度
2-8°C
SMILES 字串
Cc1ccc(C)c(OCCCC(C)(C)C(O)=O)c1
InChI
1S/C15H22O3/c1-11-6-7-12(2)13(10-11)18-9-5-8-15(3,4)14(16)17/h6-7,10H,5,8-9H2,1-4H3,(H,16,17)
InChI 密鑰
HEMJJKBWTPKOJG-UHFFFAOYSA-N
基因資訊
human ... PPARA(5465)
尋找類似的產品? 前往 產品比較指南
一般說明
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Gemfibrozil is a cholesterol-lowering drug that effectively lowers the serum cholesterol, triglyceride, and low-density lipoprotein (LDL) levels. It also efficiently raises the serum high-density lipoprotein (HDL) levels. It is known to minimize the incidence of coronary heart diseases in humans.
Gemfibrozil is a cholesterol-lowering drug that effectively lowers the serum cholesterol, triglyceride, and low-density lipoprotein (LDL) levels. It also efficiently raises the serum high-density lipoprotein (HDL) levels. It is known to minimize the incidence of coronary heart diseases in humans.
應用
Gemfibrozil may be used as a pharmaceutical reference standard for the determination of the analyte in pharmaceutical formulations by high performance liquid chromatography (HPLC).
These Secondary Standards are qualified as Certified Reference Materials. These are suitable for use in several analytical applications including but not limited to pharma release testing, pharma method development for qualitative and quantitative analyses, food and beverage quality control testing, and other calibration requirements.
分析報告
These secondary standards offer multi-traceability to the USP, EP (PhEur) and BP primary standards, where they are available.
其他說明
This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
腳註
To see an example of a Certificate of Analysis for this material enter LRAA0300 in the slot below. This is an example certificate only and may not be the lot that you receive.
相關產品
產品號碼
描述
訂價
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Development and validation of liquid chromatographic method for gemfibrozil and simvastatin in binary combination
Ashfaq M, et al.
Journal of the Chilean Chemical Society, 53(3), 1617-1619 (2008)
A M Filppula et al.
Clinical pharmacology and therapeutics, 94(3), 383-393 (2013-05-10)
Cytochrome P450 (CYP) 3A4 is considered the most important enzyme in imatinib biotransformation. In a randomized, crossover study, 10 healthy subjects were administered gemfibrozil 600 mg or placebo twice daily for 6 days, and imatinib 200 mg on day 3, to study
Ana Maria Sierra Villar et al.
International journal of pharmaceutics, 431(1-2), 161-175 (2012-04-14)
Self-nanoemulsifying drug delivery systems of gemfibrozil were developed under Quality by Design approach for improvement of dissolution and oral absorption. Preliminary screening was performed to select proper components combination. Box-Behnken experimental design was employed as statistical tool to optimize the
Manthena V S Varma et al.
Pharmaceutical research, 29(10), 2860-2873 (2012-05-29)
To develop physiologically based pharmacokinetic (PBPK) model to predict the pharmacokinetics and drug-drug interactions (DDI) of pravastatin, using the in vitro transport parameters. In vitro hepatic sinusoidal active uptake, passive diffusion and canalicular efflux intrinsic clearance values were determined using
P A Todd et al.
Drugs, 36(3), 314-339 (1988-09-01)
Gemfibrozil is a lipid-regulating agent which is generically classified as a fibric acid derivative, but which exhibits different pharmacological effects from other such drugs. Published data indicate that in patients with all types of dyslipidaemia (except type I) gemfibrozil 800
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務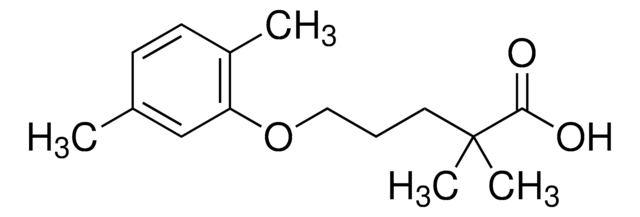



![2-[4-(4-Chlorobenzoyl)phenoxy]-2-methylpropionic acid 95%](/deepweb/assets/sigmaaldrich/product/structures/779/056/45779a0b-0c78-49b4-895b-eae07474ee2e/640/45779a0b-0c78-49b4-895b-eae07474ee2e.png)


