推薦產品
生物源
synthetic
等級
pharmaceutical primary standard
agency
EP
API 家族
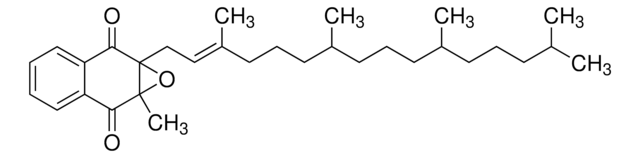
phytomenadione
形狀
solid
製造商/商標名
EDQM
技術
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
折射率
n20/D 1.527 (lit.)
mp
−20 °C (lit.)
密度
0.984 g/mL at 25 °C (lit.)
應用
pharmaceutical (small molecule)
格式
neat
儲存溫度
2-8°C
SMILES 字串
O=C(C1=CC=CC=C21)C(C)=C(C/C=C(CCC[C@@H](CCC[C@H](C)CCCC(C)C)C)\C)C2=O
InChI
1S/C31H46O2/c1-22(2)12-9-13-23(3)14-10-15-24(4)16-11-17-25(5)20-21-27-26(6)30(32)28-18-7-8-19-29(28)31(27)33/h7-8,18-20,22-24H,9-17,21H2,1-6H3/b25-20+/t23-,24-/m1/s1
InChI 密鑰
MBWXNTAXLNYFJB-NKFFZRIASA-N
尋找類似的產品? 前往 產品比較指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the Issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
Phytomenadione is a methyl napthoquinone derivative, and a naturally occurring fat soluble vitamin K1. It plays a key role in the maintenance of normal blood clotting mechanism and also in the prevention of hemorrhagic disease in newborns.
Phytomenadione is a methyl napthoquinone derivative, and a naturally occurring fat soluble vitamin K1. It plays a key role in the maintenance of normal blood clotting mechanism and also in the prevention of hemorrhagic disease in newborns.
應用
This European Pharmacopoeia reference standard is intended for use only as specifically prescribed in the European Pharmacopoeia.
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 1
閃點(°F)
Not applicable
閃點(°C)
Not applicable
客戶也查看了
Clinical Pharmacology, (11) (2012)
Development and validation of spectrophotometric methods for determination of phytomenadione in injection
Patel AS and Parikh KP
Current Research in Pharmaceutical Sciences, 26-30 (2012)
Bernard Laubscher et al.
European journal of pediatrics, 172(3), 357-360 (2012-11-30)
In 2003, the Swiss guidelines to prevent vitamin K deficiency bleeding (VKDB) were adapted. As two oral doses (2 mg, hour/day 4) of mixed micellar VK preparation had failed to abolish late VKDB, a third dose (week 4) was introduced.
Ellen C M Cranenburg et al.
Kidney international, 82(5), 605-610 (2012-06-01)
Vitamin K is essential for the activity of γ-carboxyglutamate (Gla)-proteins including matrix Gla28 protein and osteocalcin; an inhibitor of vascular calcification and a bone matrix protein, respectively. Insufficient vitamin K intake leads to the production of non-carboxylated, inactive proteins and
Rogier Caluwé et al.
Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association, 29(7), 1385-1390 (2013-11-29)
Haemodialysis patients suffer from accelerated vascular calcification. The vitamin K-dependent matrix Gla protein (MGP) is one of the most powerful inhibitors of vascular calcification. Haemodialysis patients have high levels of the inactive form of MGP (desphosphorylated-uncarboxylated-MGP, dp-uc-MGP) and may benefit
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務