推薦產品
等級
purum
品質等級
化驗
≥96.5% (HPLC)
形狀
powder
mp
119-122 °C (lit.)
120-124 °C
官能基
ketone
SMILES 字串
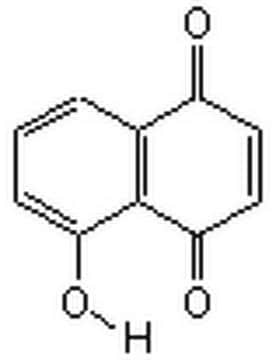
O=C1C=CC(=O)c2ccccc12
InChI
1S/C10H6O2/c11-9-5-6-10(12)8-4-2-1-3-7(8)9/h1-6H
InChI 密鑰
FRASJONUBLZVQX-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
應用
1,4-Naphthoquinone is the key structural moiety of many anticancer and antifungal agents.[1]
It can be used to synthesize:[2]
Additional appilcation include:[2]
It can be used to synthesize:[2]
- 3,3-Disubstituted oxindoles via asymmetric Michael addition to oxindole.
- Bioactive isoindolines via asymmetric 1,3-dipolar cycloaddition to azomethine ylides generated in situ from aldehydes and diethyl aminomalonate.
- α,α-Difluoro-β-hydroxy ketone via ‘on water′ catalyst-free Mukaiyama-aldol reaction with difluoroenoxysilane.
- 2-Hydroxy-3-anilino-1,4-naphthoquinone, which shows potent in vivo antimalarial activity.
Additional appilcation include:[2]
- As an arylation reagent for the α-arylation of aldehydes.
- As a starting material in the multi-step synthesis of benz[f]indole-4,9-diones.
訊號詞
Danger
危險分類
Acute Tox. 1 Inhalation - Acute Tox. 3 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Dam. 1 - Skin Corr. 1C - Skin Sens. 1 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
285.8 °F
閃點(°C)
141 °C
個人防護裝備
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
客戶也查看了
1, 4-Naphthoquinone.
Yu J S.
Synlett, 25(16), 2377-2378 (2014)
Jan Bitenc et al.
Materials (Basel, Switzerland), 13(3) (2020-01-25)
Organic cathode materials are promising cathode materials for multivalent batteries. Among organic cathodes, anthraquinone (AQ) has already been applied to various metal‒organic systems. In this work, we compare electrochemical performance and redox potential of AQ with 1,4-naphthoquinone (NQ) and 1,4-benzoquinone
Michael G Berg et al.
Molecular and cellular biology, 32(7), 1271-1283 (2012-01-19)
Despite intensive research, there are very few reagents with which to modulate and dissect the mRNA splicing pathway. Here, we describe a novel approach to identify such tools, based on detection of the exon junction complex (EJC), a unique molecular
Don Antoine Lanfranchi et al.
Organic & biomolecular chemistry, 10(31), 6375-6387 (2012-07-11)
Improving the solubility of polysubstituted 1,4-naphthoquinone derivatives was achieved by introducing nitrogen in two different positions of the naphthoquinone core, at C-5 and at C-8 of menadione through a two-step, straightforward synthesis based on the regioselective hetero-Diels-Alder reaction. The antimalarial
Design, synthesis and evaluation of novel 1, 4-naphthoquinone derivatives as antifungal and anticancer agents.
Tandon V K, et al.
Bioorganic & Medicinal Chemistry Letters, 14(5), 1079-1083 (2004)
Active Filters
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務