919896
C5 Lenalidomide-dipiperazine-NH2 hydrochloride
同義詞:
4-(4-(2-Aminoethyl)piperazine-1-carbonyl)-N-(2-(2,6-dioxopiperidin-3-yl)-1-oxoisoindolin-5-yl)piperazine-1-carboxamide, C5 Lenalidomide conjugate, Crosslinker−E3 Ligase ligand conjugate, Protein degrader building block for PROTAC® research, Template for synthesis of targeted protein degrader
About This Item
推薦產品
ligand
C5 Lenalidomide
品質等級
形狀
solid
反應適用性
reactivity: carboxyl reactive
reagent type: ligand-linker conjugate
儲存溫度
2-8°C
SMILES 字串
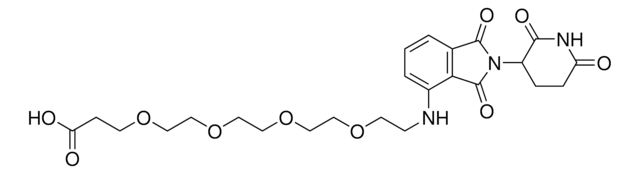
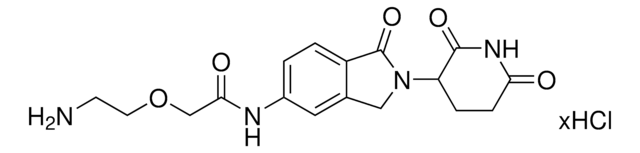
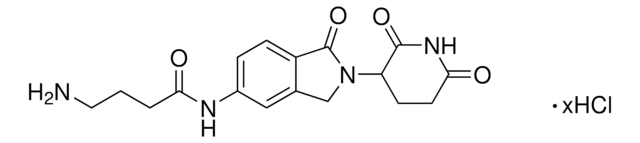
O=C(N(CC1)CCN1C(NC2=CC(C3)=C(C=C2)C(N3C(C4=O)CCC(N4)=O)=O)=O)N5CCN(CC5)CCN.Cl
應用
其他說明
Portal: Building PROTAC® Degraders for Targeted Protein Degradation
Targeted Protein Degradation by Small Molecules
Small-Molecule PROTACS: New Approaches to Protein Degradation
Targeted Protein Degradation: from Chemical Biology to Drug Discovery
法律資訊
相關產品
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
文章
Protein Degrader Building Blocks 是一系列交聯劑-E3 配體結合物,具有懸垂功能基團,可與目標配體共價連結。
Protein Degrader Building Blocks are a collection of crosslinker-E3 ligand conjugates with a pendant functional group for covalent linkage to a target ligand.
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務
![Exo-Phenyl Kwon [2.2.1]双环膦 95% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/477/026/5255f657-4af5-47da-9839-86b94d92129f/640/5255f657-4af5-47da-9839-86b94d92129f.png)
![Exo-4-anisole Kwon [2.2.1] bicyclic phosphine](/deepweb/assets/sigmaaldrich/product/structures/114/753/2a544671-b0e0-4556-8dc3-46c126d6c8ab/640/2a544671-b0e0-4556-8dc3-46c126d6c8ab.png)