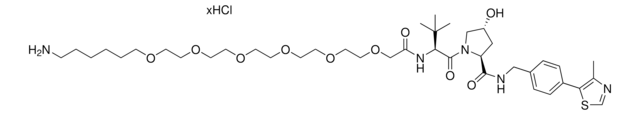
901511
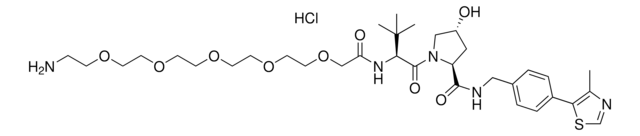
(S,R,S)-AHPC-PEG3-NH2 hydrochloride
≥95%
同義詞:
(2S,4R)-1-((S)-14-Amino-2-(tert-butyl)-4-oxo-6,9,12-trioxa-3-azatetradecanoyl)-4-hydroxy-N-(4-(4-methylthiazol-5-yl)benzyl)pyrrolidine-2-carboxamide hydrochloride, Crosslinker–E3 Ligase ligand conjugate, Protein degrader building block for PROTAC® research, Template for synthesis of targeted protein degrader, VH032 conjugate
About This Item
推薦產品
ligand
VH032
品質等級
化驗
≥95%
形狀
powder or crystals
反應適用性
reactivity: carboxyl reactive
reagent type: ligand-linker conjugate
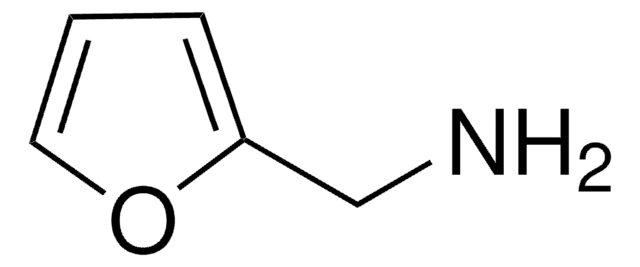
官能基
amine
儲存溫度
2-8°C
SMILES 字串
O=C(NCC1=CC=C(C2=C(C)N=CS2)C=C1)[C@H](C[C@@H](O)C3)N3C([C@H](C(C)(C)C)NC(COCCOCCOCCN)=O)=O.Cl
InChI 密鑰
ZOYHUTRHKHRRPK-QVRKWNSCSA-N
應用
Automate your VHL-PEG based PROTACs with Synple Automated Synthesis Platform (SYNPLE-SC002)
其他說明
Portal: Building PROTAC® Degraders for Targeted Protein Degradation
Impact of Target Warhead and Linkage Vector on Inducing Protein Degradation: Comparison of Bromodomain and Extra-Terminal (BET) Degraders Derived from Triazolodiazepine (JQ1) and Tetrahydroquinoline (I-BET726) BET Inhibitor Scaffolds
Degradation of the BAF Complex Factor BRD9 by Heterobifunctional Ligands
Assessing Different E3 Ligases for Small Molecule Induced Protein Ubiquitination and Degradation
Targeted Protein Degradation by Small Molecules
Impact of linker length on the activity of PROTACs
法律資訊
相關產品
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
從最近期的版本中選擇一個:
分析證明 (COA)
客戶也查看了
文章
Protein Degrader Building Blocks are a collection of crosslinker-E3 ligand conjugates with a pendant functional group for covalent linkage to a target ligand.
Protein Degrader Building Blocks 是一系列交聯劑-E3 配體結合物,具有懸垂功能基團,可與目標配體共價連結。
Active Filters
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務